
Asia Pacific Drug Development Market Outlook to 2030
Region:Asia
Author(s):Naman Rohilla
Product Code:KROD6858
December 2024
81
About the Report
Asia Pacific Drug Development Market Overview
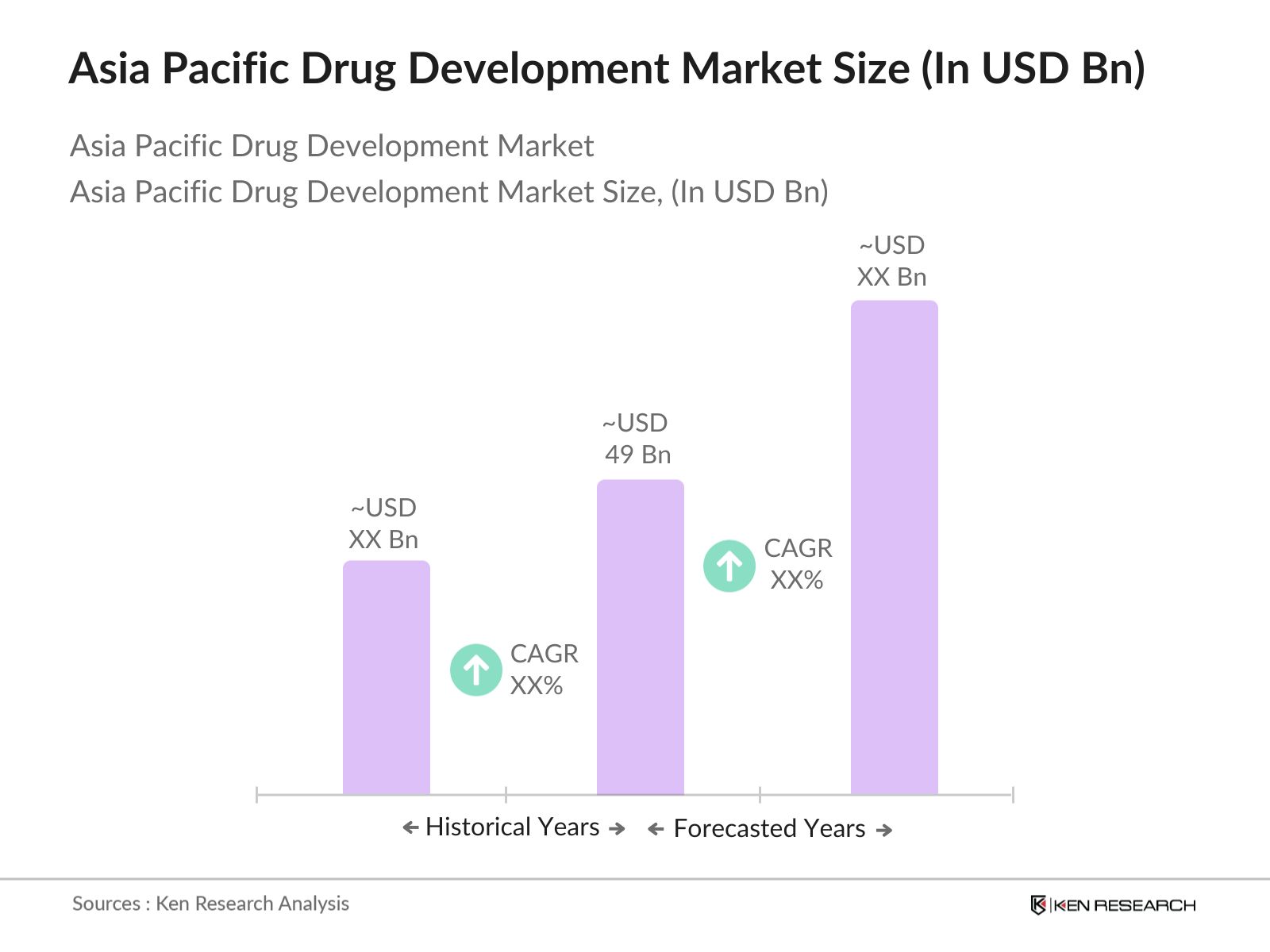
- The Asia Pacific drug development market is valued at USD 49 billion, based on a five-year historical analysis. This market is driven by rapid advancements in drug discovery technologies, including AI-based drug discovery platforms, increasing R&D investments by pharmaceutical companies, and rising demand for innovative therapies across various therapeutic areas. The growing focus on precision medicine and personalized treatments is further accelerating the growth of the drug development market in the region, with contributions from both small molecules and biologics.
- Several countries dominate the Asia Pacific drug development market due to their strong pharmaceutical industries, robust research ecosystems, and government support. China, India, and Japan lead the market, driven by their advanced manufacturing capabilities, investment in biopharmaceutical research, and extensive clinical trial activities. Chinas dominance is further supported by its growing investment in biotechnology, while Japan is a hub for innovation, particularly in biologics and regenerative medicine. Indias strength lies in its cost-effective clinical trials and production of generic drugs.
- The drug approval processes in the Asia Pacific region are characterized by varying regulatory frameworks. For instance, Japans PMDA and Chinas CFDA have introduced expedited approval pathways for innovative drugs, reducing approval times to as little as 9 months. In comparison, the U.S. FDA takes an average of 12 months for drug approval. In 2024, Chinas CFDA approved over 300 innovative drugs, signalling a more competitive and streamlined regulatory environment. This regulatory alignment across the region is fostering a faster market entry for novel therapies.
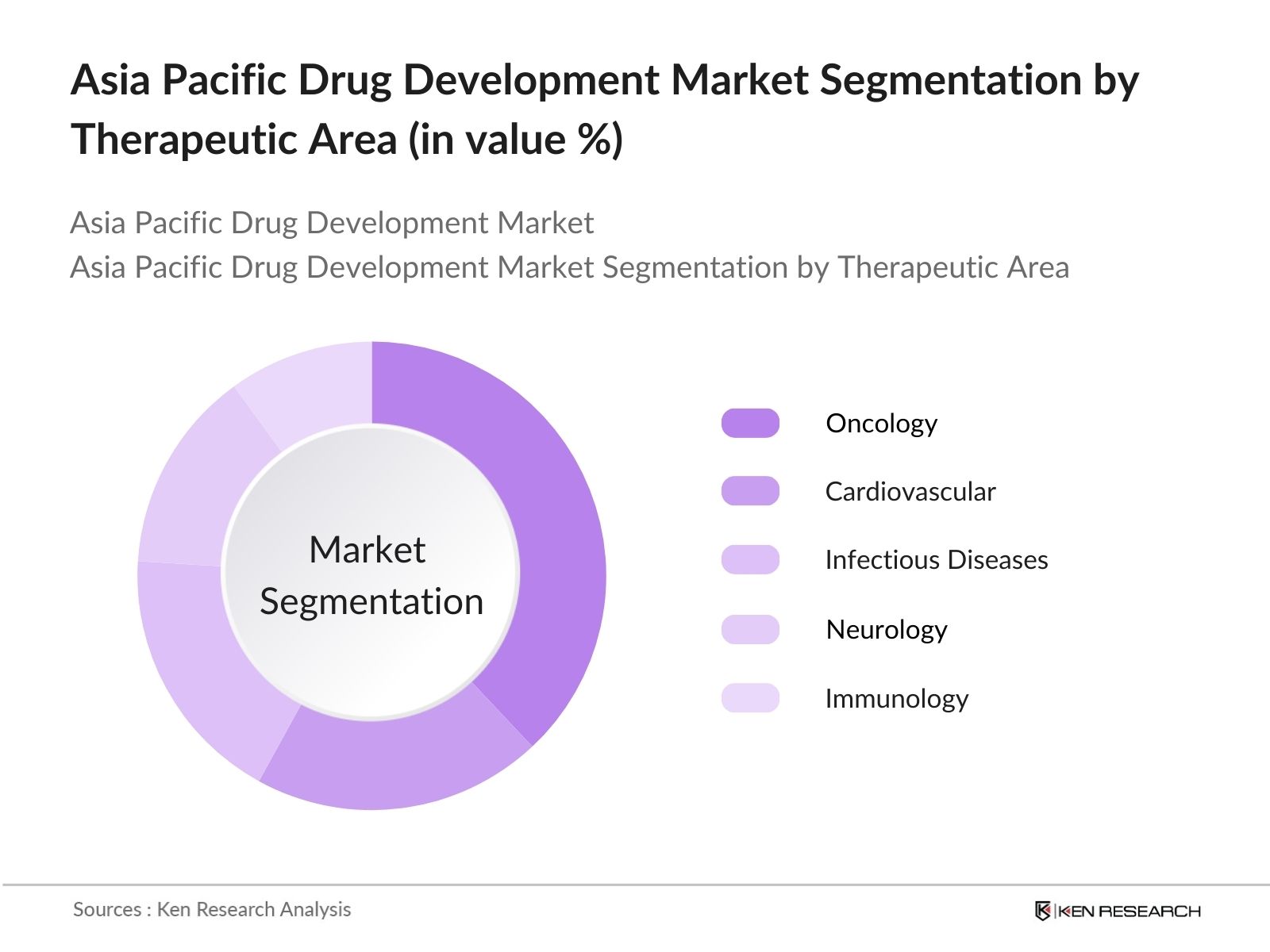
Asia Pacific Drug Development Market Segmentation
- By Therapeutic Area: The Asia Pacific drug development market is segmented by therapeutic area into oncology, cardiovascular, infectious diseases, neurology, and immunology. Oncology holds the dominant market share within this segment due to the high prevalence of cancer across the region and the increasing focus on developing novel cancer therapies. The rising demand for targeted therapies, immuno-oncology, and combination treatments is driving significant R&D investments in oncology, particularly in China and Japan, where clinical trials and drug development efforts are robust.
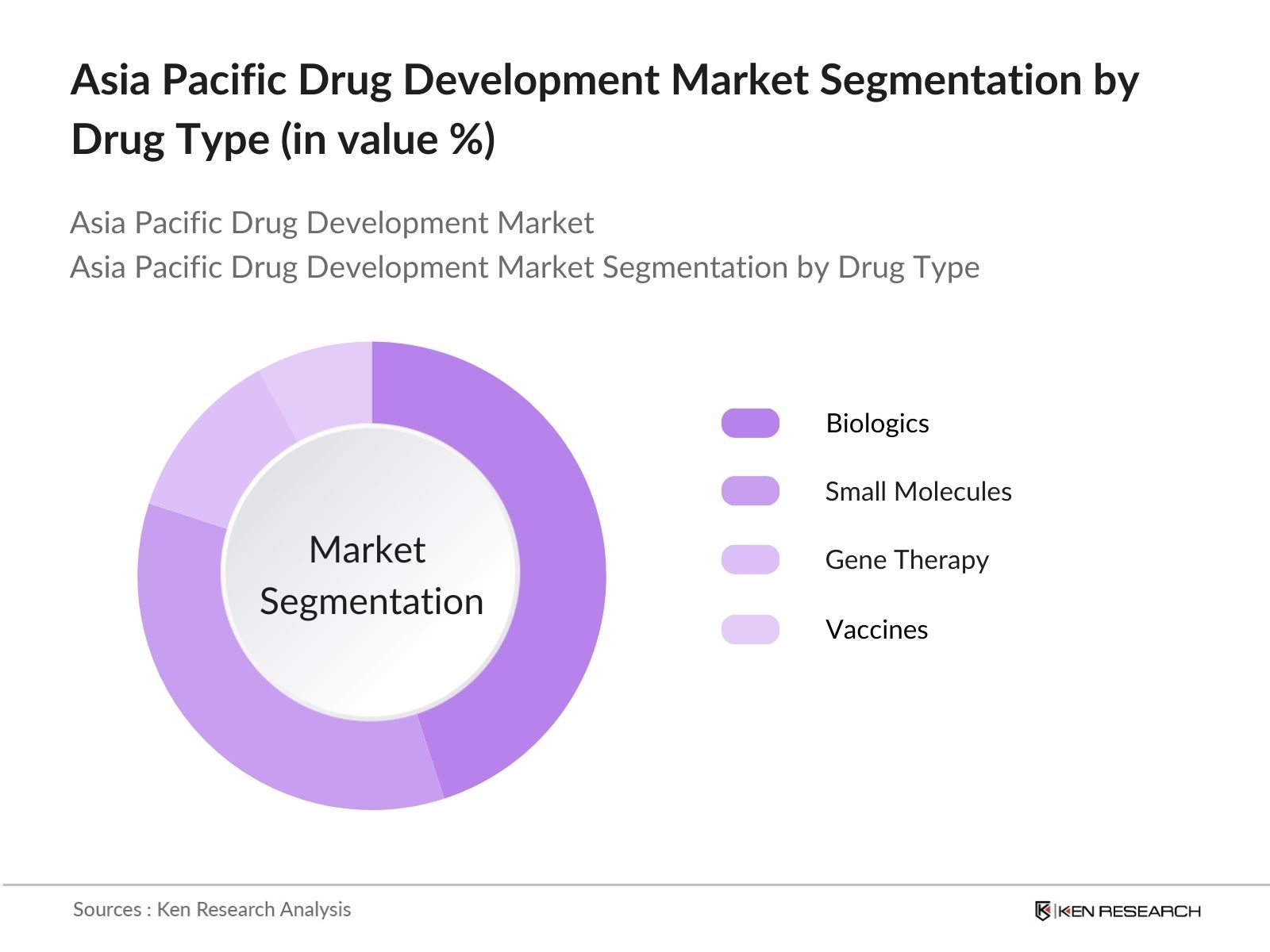
- By Drug Type: The market is also segmented by drug type into small molecules, biologics, gene therapy, and vaccines. Biologics hold a dominant share of the market, driven by the rapid advancements in biotechnology and the growing demand for monoclonal antibodies, cell and gene therapies, and other biologics. The biopharmaceutical industry is expanding significantly in the Asia Pacific region, with China and India emerging as major hubs for biologic manufacturing and development due to their cost advantages and supportive government policies.
Asia Pacific Drug Development Market Competitive Landscape
The Asia Pacific drug development market is dominated by both local and international players, with several companies leading in innovation, clinical trials, and manufacturing capabilities. Companies such as Novartis, Pfizer, and Takeda Pharmaceutical have strong R&D pipelines, strategic partnerships, and a global reach, further consolidating their positions in the market. Additionally, local firms like WuXi AppTec and Biocon are gaining ground due to their cost-efficient research and production capabilities.
|
Company |
Establishment Year |
Headquarters |
Market Presence |
R&D Expenditure |
Product Portfolio |
Partnerships |
Patents |
Global Reach |
Innovations |
|
Novartis AG |
1996 |
Basel, Switzerland |
- |
- |
- |
- |
- |
- |
- |
|
Pfizer Inc. |
1849 |
New York, USA |
- |
- |
- |
- |
- |
- |
- |
|
Roche Holding AG |
1896 |
Basel, Switzerland |
- |
- |
- |
- |
- |
- |
- |
|
Takeda Pharmaceutical Co. |
1781 |
Tokyo, Japan |
- |
- |
- |
- |
- |
- |
- |
|
WuXi AppTec |
2000 |
Shanghai, China |
- |
- |
- |
- |
- |
- |
- |
Asia Pacific Drug Development Market Analysis
Asia Pacific Drug Development Market Growth Drivers
- Increase in Clinical Trials: The Asia Pacific region has witnessed a notable rise in the volume of clinical trials over the past few years. According to the World Health Organization's Global Clinical Trials Registry, as of 2024, more than 25,000 clinical trials are ongoing across the region, especially in countries like China, Japan, and South Korea. The trial success rate is also improving, with an increase of 5% in the past three years, driven by technological innovations. This rise is due to enhanced research capacities and growing collaborations with academic institutions in the drug discovery process.
- Advances in Drug Discovery: Advances in drug discovery are being fueled by the incorporation of AI and machine learning, which is expected to accelerate drug development timelines. In 2024, AI-driven platforms have been reported to cut early-stage drug discovery time by nearly 15% compared to traditional methods, according to the International Federation of Pharmaceutical Manufacturers & Associations. These technologies are being widely adopted in leading countries like Japan, where AI is playing a key role in streamlining target identification processes and optimizing compound selection, resulting in higher success rates in clinical trials.
- Rising Healthcare Expenditure: Healthcare expenditure is on the rise in the Asia Pacific region, with total healthcare spending across China, India, and Japan surpassing $3.5 trillion in 2024, according to the World Bank. Governments are channelling a portion of this expenditure toward drug R&D, with China alone dedicating $1.4 trillion to healthcare, of which $150 billion is allocated to pharmaceutical research. This surge in investment is driving innovation, particularly in biopharmaceuticals, with increased funding towards R&D initiatives and infrastructure development.
Asia Pacific Drug Development Market Challenges
- High Drug Development Costs: The high cost of drug development continues to pose a major challenge in the Asia Pacific market. According to a 2023 World Bank report, the average cost of developing a new drug range from $2 billion to $2.6 billion, making it difficult for smaller firms to compete in the market. Furthermore, delays in drug trials and approval processes further add to these costs. In countries like Australia and Japan, drug development costs are particularly high due to stringent regulations and lengthy approval processes, which contribute to the financial burden faced by pharmaceutical companies.
- Regulatory Hurdles: Navigating the complex regulatory landscape in the Asia Pacific region remains a challenge for drug developers. In 2024, the average drug approval timeline in major markets like China and India ranges from 12 to 18 months, according to the OECD, while markets like South Korea offer expedited pathways for innovative drugs. These timelines impact the market entry and profitability of new therapies. Regulatory inconsistencies between countries further complicate the process, requiring companies to tailor strategies for each market.
Asia Pacific Drug Development Market Future Outlook
Over the next five years, the Asia Pacific drug development market is expected to show growth driven by the continuous development of biologics and gene therapies, increased R&D investments, and an expanding pipeline of innovative drugs. The region's evolving regulatory landscape, particularly the acceleration of drug approvals in countries like China and Japan, will further propel the market. Additionally, the increasing demand for precision medicine and the growing focus on rare diseases will create new opportunities for market players.
Asia Pacific Drug Development Market Opportunities
- Growing Demand for Personalized Medicine: There is an increasing demand for personalized medicine in the Asia Pacific region, particularly in precision oncology and rare disease treatments. As of 2024, Japan and China have seen a 30% rise in personalized medicine clinical trials over the past three years, according to the World Health Organization. The adoption of biomarker-driven therapies is driving this demand, with significant investments in genomic research. This trend is expected to expand as more healthcare providers and regulatory bodies prioritize precision medicine to enhance patient outcomes and reduce healthcare costs.
- Expansion of Biosimilars Market: The Asia Pacific region has become a hub for biosimilar development, particularly in countries like India, South Korea, and China, which have seen a 40% rise in biosimilar approvals in 2023, according to the European Medicines Agency. This growth is driven by favourable government policies, reduced regulatory barriers, and increasing healthcare expenditures. South Korea, for example, has invested $350 million in biosimilar R&D, positioning itself as a leading global biosimilar producer, while India has over 60 approved biosimilars, with plans for further expansion in the coming years.
Scope of the Report
|
Therapeutic Area |
Oncology Cardiovascular Infectious Diseases Neurology Immunology |
|
Drug Type |
Small Molecules Biologics Gene Therapy Vaccines |
|
Phase of Development |
Preclinical Phase I Phase II Phase III |
|
End User |
Pharmaceutical Companies CROs Academic Institutions Government Organizations |
|
Country |
China India Japan South Korea Australia |
Products
Key Target Audience
Pharmaceutical Manufacturers
Biopharmaceutical Companies
Contract Research Organizations (CROs)
Drug Regulatory Authorities (FDA, PMDA, CFDA)
Banks and Financial Institutions
Government and Regulatory Bodies (China National Medical Products Administration, Japan Pharmaceuticals and Medical Devices Agency)
Biotechnology Firms
Investor and Venture Capitalist Firms
Academic and Research Institutes
Companies
Players Mentioned in the Report
Novartis AG
Pfizer Inc.
Roche Holding AG
Takeda Pharmaceutical Company
Astellas Pharma
Biocon
Dr. Reddys Laboratories
WuXi AppTec
ICON Plc
Table of Contents
1. Asia Pacific Drug Development Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. Asia Pacific Drug Development Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. Asia Pacific Drug Development Market Analysis
3.1. Growth Drivers
3.1.1. Increase in Clinical Trials (Volume of Trials, Trial Success Rates, Innovation)
3.1.2. Advances in Drug Discovery (AI and ML Applications)
3.1.3. Regulatory Support for Biopharma (Government Initiatives and Funding)
3.1.4. Rising Healthcare Expenditure (Healthcare Investment and R&D Expenditure)
3.2. Market Challenges
3.2.1. High Drug Development Costs
3.2.2. Regulatory Hurdles (Market Access and Drug Approval Timelines)
3.2.3. Scarcity of Skilled Workforce
3.3. Opportunities
3.3.1. Growing Demand for Personalized Medicine
3.3.2. Expansion of Biosimilars Market
3.3.3. Collaborations and Partnerships in Drug Discovery
3.4. Trends
3.4.1. Increasing Use of Biomarkers in Drug Development
3.4.2. Shift to Small Molecule Targeted Therapies
3.4.3. Adoption of Blockchain in Drug Development Processes
3.5. Regulatory Landscape
3.5.1. Regional Drug Approval Processes (FDA, PMDA, CFDA)
3.5.2. Fast-Track Approvals and Priority Review Pathways
3.5.3. Intellectual Property Protection for New Drugs
3.6. SWOT Analysis
3.7. Ecosystem Analysis (Pharma, CROs, CMOs, Academia)
3.8. Porters Five Forces Analysis
3.9. Competition Ecosystem
4. Asia Pacific Drug Development Market Segmentation
4.1. By Therapeutic Area (In Value %)
4.1.1. Oncology
4.1.2. Cardiovascular
4.1.3. Infectious Diseases
4.1.4. Neurology
4.1.5. Immunology
4.2. By Drug Type (In Value %)
4.2.1. Small Molecules
4.2.2. Biologics
4.2.3. Gene Therapy
4.2.4. Vaccines
4.3. By Phase of Development (In Value %)
4.3.1. Preclinical
4.3.2. Phase I
4.3.3. Phase II
4.3.4. Phase III
4.4. By End User (In Value %)
4.4.1. Pharmaceutical Companies
4.4.2. Contract Research Organizations (CROs)
4.4.3. Academic and Research Institutions
4.4.4. Government Organizations
4.5. By Country (In Value %)
4.5.1. China
4.5.2. India
4.5.3. Japan
4.5.4. South Korea
4.5.5. Australia
5. Asia Pacific Drug Development Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Novartis AG
5.1.2. Pfizer Inc.
5.1.3. Roche Holding AG
5.1.4. GSK Plc
5.1.5. Merck & Co.
5.1.6. AstraZeneca
5.1.7. Johnson & Johnson
5.1.8. Sanofi
5.1.9. Takeda Pharmaceutical Company
5.1.10. Astellas Pharma
5.1.11. Biocon
5.1.12. Dr. Reddy's Laboratories
5.1.13. WuXi AppTec
5.1.14. ICON Plc
5.1.15. Parexel International
5.2. Cross Comparison Parameters (R&D Spend, Market Share, Product Pipeline, Drug Approval Success Rate, Manufacturing Capabilities, Global Reach, Patents Filed, Partnerships and Collaborations)
5.3. Market Share Analysis
5.4. Strategic Initiatives (Acquisitions, Joint Ventures, Strategic Alliances)
5.5. Mergers and Acquisitions
5.6. Investment Analysis (Public and Private Funding)
5.7. Venture Capital and Private Equity Investments
5.8. Government Grants and Incentives
6. Asia Pacific Drug Development Market Regulatory Framework
6.1. Regional Compliance Standards (FDA, PMDA, CFDA)
6.2. Drug Pricing Regulations
6.3. Certifications and Quality Standards (GMP, GLP, GCP)
7. Asia Pacific Drug Development Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. Asia Pacific Drug Development Future Market Segmentation
8.1. By Therapeutic Area (In Value %)
8.2. By Drug Type (In Value %)
8.3. By Phase of Development (In Value %)
8.4. By End User (In Value %)
8.5. By Country (In Value %)
9. Asia Pacific Drug Development Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Strategic Market Entry Options
9.3. Customer Segmentation Insights
9.4. Investment Recommendations
Research Methodology
Step 1: Identification of Key Variables
In this phase, we identify and define key variables influencing the Asia Pacific drug development market by analyzing the ecosystem, key stakeholders, and trends. Extensive desk research using industry databases helps gather market insights for understanding market dynamics.
Step 2: Market Analysis and Construction
This step includes gathering historical data on drug development in the region. We assess clinical trials, R&D expenditures, and regulatory milestones to construct the markets overall structure. This helps to analyze market performance and segmentation accurately.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are validated by conducting interviews with experts from pharmaceutical companies and regulatory bodies. These insights help refine market estimates and provide firsthand perspectives on market drivers and challenges.
Step 4: Research Synthesis and Final Output
The final phase involves synthesizing the research findings, validating them through multiple industry sources, and preparing the final output. This process ensures a comprehensive and reliable analysis of the Asia Pacific drug development market.
Frequently Asked Questions
01. How big is the Asia Pacific Drug Development Market?
The Asia Pacific drug development market is valued at USD 49 billion, driven by the growing need for innovative therapies, R&D investments, and advancements in drug discovery technologies.
02. What are the challenges in the Asia Pacific Drug Development Market?
Challenges in the Asia Pacific drug development market include high drug development costs, regulatory hurdles, and a shortage of skilled professionals in the region. Additionally, intellectual property protection can present obstacles to innovation.
03. Who are the major players in the Asia Pacific Drug Development Market?
Major players in the Asia Pacific drug development market include Novartis AG, Pfizer Inc., Roche Holding AG, Takeda Pharmaceutical Company, and WuXi AppTec, dominating due to their extensive R&D capabilities, global reach, and diverse product portfolios.
04. What drives the growth of the Asia Pacific Drug Development Market?
The Asia Pacific drug development market is propelled by advancements in biotechnology, the growing prevalence of chronic diseases, increasing demand for biologics, and government support for drug development activities across the region.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















