
Global Herpangina Treatment Market Outlook to 2030
Region:Global
Author(s):Meenakshi Bisht
Product Code:KROD8155
December 2024
86
About the Report
Global Herpangina Treatment Market Overview
- The Global Herpangina Treatment Market is currently valued at USD 1.65 billion, driven by rising incidences of viral infections among pediatric populations and growing awareness of viral disease treatments. The market has been growing steadily due to increased access to healthcare in emerging markets and ongoing efforts by governments to enhance pediatric healthcare infrastructure.
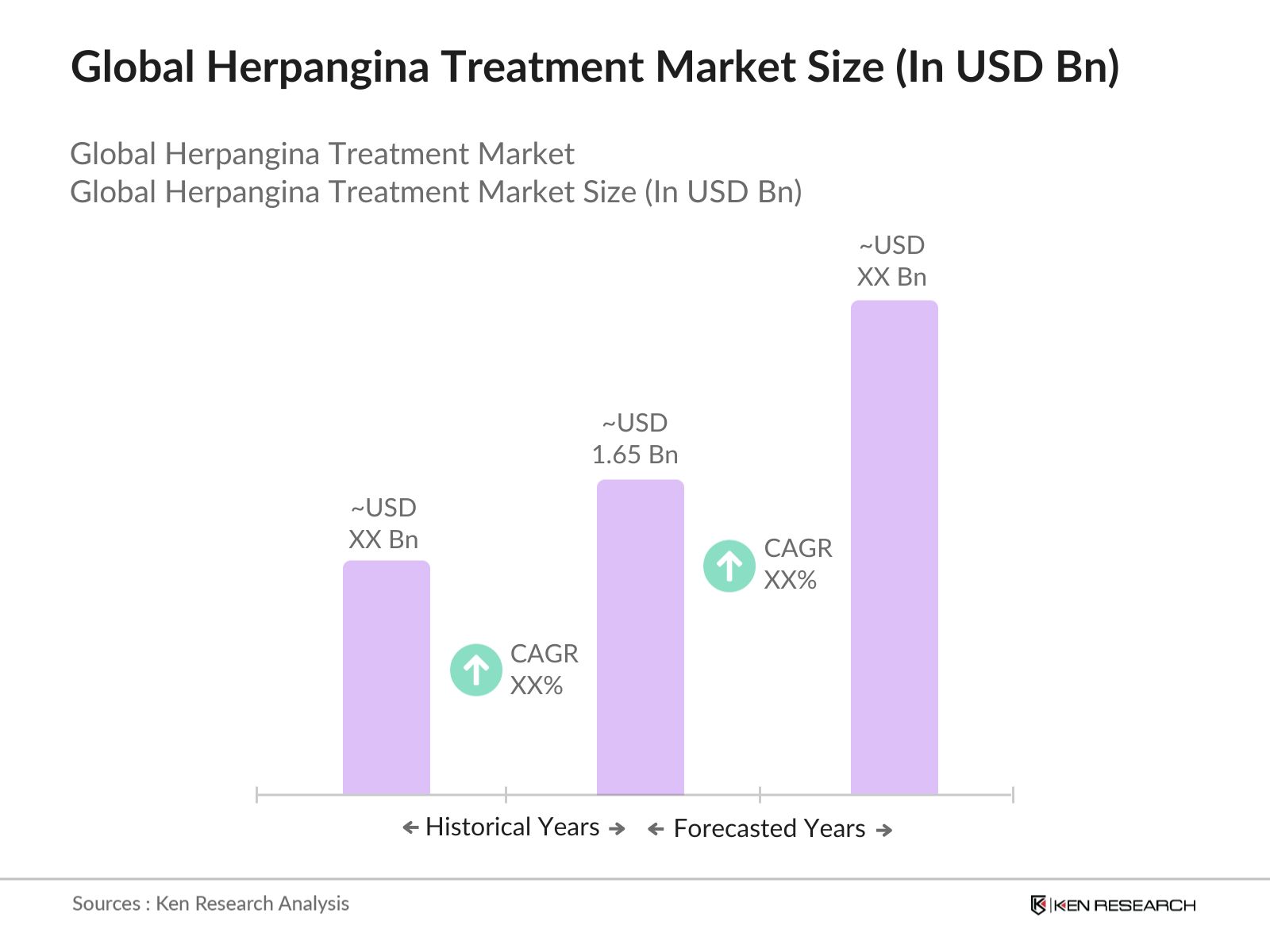
- Countries such as the United States, China, and India dominate the herpangina treatment market due to their vast pediatric population, well-established healthcare systems, and the presence of major pharmaceutical companies. The dominance of these countries is also fueled by their increasing investments in healthcare infrastructure and the rising incidence of viral infections in densely populated regions. The availability of advanced diagnostic tools in these countries further strengthens their position in the market.
- Several countries have introduced mandatory reporting of viral infections, including pediatric cases of herpangina. In 2023, the United States, Canada, and several European nations updated their healthcare regulations to include compulsory reporting of all viral infections in children. This regulatory framework aims to improve data collection, monitor disease outbreaks, and facilitate early intervention. Mandatory reporting is expected to enhance the tracking and management of herpangina, contributing to better healthcare outcomes for affected children.
Global Herpangina Treatment Market Segmentation
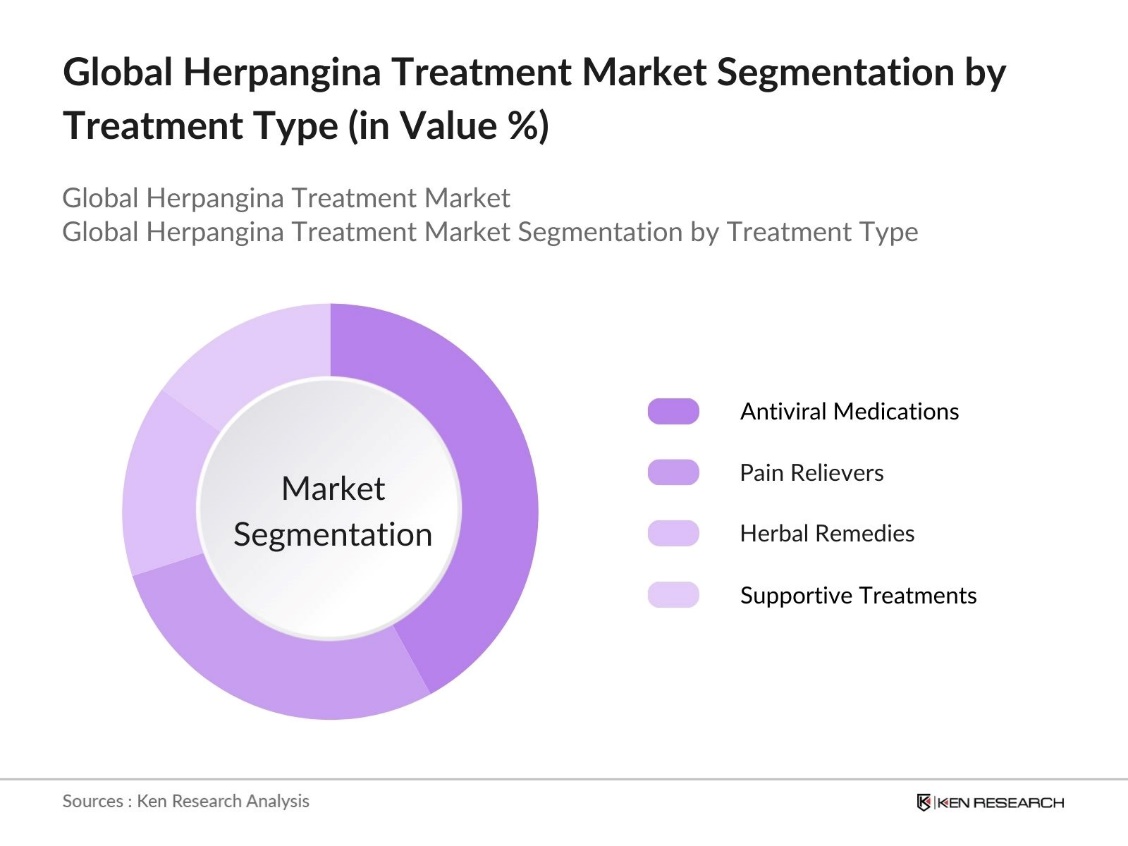
By Treatment Type: The herpangina treatment market is segmented into antiviral medications, pain relievers, herbal remedies, and other supportive treatments. Among these, antiviral medications hold the largest market share. This dominance can be attributed to the efficacy of antiviral drugs in managing symptoms and reducing recovery time in patients. These medications are commonly prescribed by pediatricians worldwide, leading to consistent demand in both developed and developing regions.
By Patient Age Group: The market is segmented by patient age into pediatric (0-5 years), pediatric (6-12 years), and pediatric (13-18 years). The pediatric (0-5 years) group dominates this segment, accounting for the largest market share. This is because younger children are more susceptible to viral infections like herpangina due to their developing immune systems. Pediatric healthcare providers prioritize early detection and treatment in this age group, which drives demand for herpangina treatments.
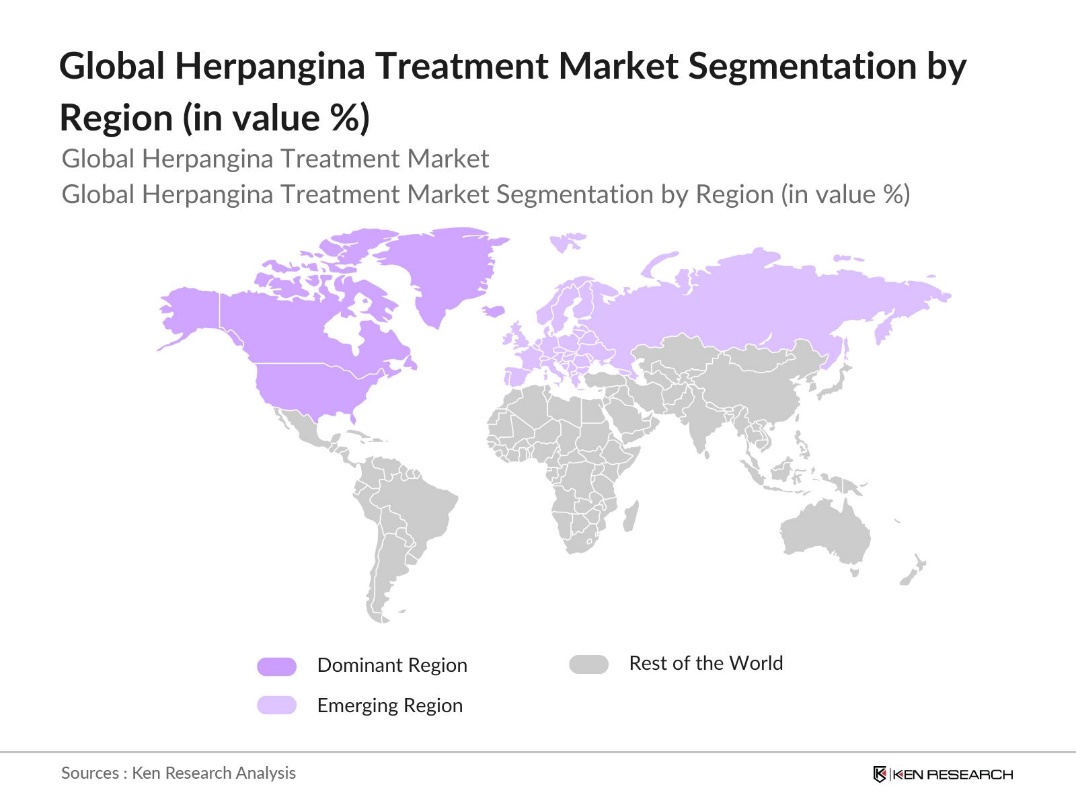
By Region: Geographically, the market is segmented into North America, Europe, Asia Pacific, Latin America, and the Middle East & Africa. North America is currently the leading region in terms of market share, primarily due to its advanced healthcare infrastructure, high healthcare spending, and widespread adoption of antiviral medications. The region benefits from strong pharmaceutical research and development efforts and high awareness among healthcare professionals regarding the treatment of viral infections in children.
Global Herpangina Treatment Market Competitive Landscape
The global herpangina treatment market is characterized by the presence of several key players who hold significant market share due to their extensive research and development capabilities, strong distribution networks, and global reach. The competitive landscape is influenced by factors such as mergers and acquisitions, product innovations, and strategic collaborations.

Global Herpangina Treatment Industry Analysis
Growth Drivers
- Rising Pediatric Population: The global pediatric population is witnessing consistent growth, particularly in low- and middle-income countries (LMICs). As of 2024, the World Bank reports over 25.02% children aged 0-14 globally, with regions such as Sub-Saharan Africa and South Asia seeing the highest concentration. This demographic growth increases the incidence of pediatric diseases, including herpangina, thus driving the demand for treatments. As healthcare systems in these regions aim to address pediatric health, the need for antiviral therapies specific to this age group is expected to surge, necessitating investments in research and development for pediatric healthcare.
- Increasing Awareness of Viral Infections: With rising global health campaigns, there is growing awareness regarding viral infections, particularly those affecting children, such as herpangina. For instance, UNICEF reported that they reached 9.3 million children with life-saving treatment for severe wasting in 2023. The growing focus on pediatric health by governments and non-governmental organizations (NGOs) highlights the increasing demand for herpangina treatments, pushing healthcare systems to invest in education and antiviral treatments.
- Improvements in Diagnostic Technologies: Advancements in diagnostic technologies, particularly in low-income regions, have improved the detection of viral infections like herpangina. The introduction of point-of-care diagnostics enables earlier identification of infections, especially in underserved areas, facilitating quicker treatment. This progress is driving the demand for targeted herpangina therapies, as healthcare systems can now more effectively manage outbreaks and reduce the impact of viral diseases in pediatric populations.
Market Challenges
- Limited Diagnostic Facilities in Rural Areas: A significant challenge for the herpangina treatment market is the lack of diagnostic infrastructure in rural regions. Limited access to healthcare facilities in these areas hampers early detection of herpangina, delaying treatment and increasing the risk of disease spread. Efforts are being made to improve healthcare accessibility, but the absence of adequate diagnostic resources remains a considerable barrier to timely treatment.
- Lack of Effective Antiviral Treatments: Another challenge in treating herpangina is the lack of effective antiviral therapies specifically targeting the disease. Many antiviral medications currently available are designed for broader viral infections and may not effectively treat herpangina. This gap in treatment options poses difficulties for healthcare providers, particularly in areas with a high incidence of pediatric cases, highlighting the need for more targeted and effective therapies.
Global Herpangina Treatment Market Future Outlook
The global herpangina treatment market is expected to witness considerable growth in the coming years, driven by the increasing prevalence of viral infections, advancements in treatment options, and enhanced healthcare infrastructure in emerging economies. As pediatric populations continue to grow, particularly in developing countries, demand for effective herpangina treatments is likely to rise. Additionally, ongoing research into antiviral medications and the potential development of vaccines could further bolster the market's growth trajectory.
Market Opportunities
- Development of Novel Antiviral Therapies: The development of novel antiviral therapies presents a major opportunity for the global herpangina treatment market. Ongoing research supported by international health organizations and governments is focused on addressing resistance to existing treatments. This opens avenues for pharmaceutical companies to create more effective antiviral therapies specifically targeting herpangina, especially in regions with large pediatric populations, where the demand for specialized treatments is growing.
- Rising Telemedicine Adoption in Developing Markets: Telemedicine adoption is growing rapidly in developing markets, particularly in response to increased demand for remote healthcare solutions. Telemedicine platforms are helping to bridge the gap in healthcare access, especially in rural and underserved areas, where diagnostic and treatment services for pediatric viral infections like herpangina are limited. This trend provides a promising platform for diagnosing and managing viral infections, driving growth potential for the herpangina treatment market as telemedicine continues to expand.
Scope of the Report
|
Treatment Type |
Antiviral Medications Pain Relievers Herbal Remedies Other Supportive Treatments |
|
Patient Age Group |
Pediatric (0-5 years) Pediatric (6-12 years) Pediatric (13-18 years) |
|
Distribution Channel |
Hospitals Retail Pharmacies Online Pharmacies Drug Stores |
|
Diagnosis Method |
Clinical Examination Lab Testing (PCR, Viral Cultures) |
|
Region |
North America Europe Asia Pacific Latin America Middle East & Africa |
Products
Key Target Audience
Pharmaceutical Companies
Digital Health Platforms
Pediatric Medical Equipment Manufacturers
Pharmaceutical Raw Material Suppliers
Government and Regulatory Bodies (e.g., FDA, EMA)
Investors and venture capital Firms
Banks and Financial Institutions
Companies
Players Mentioned in the Report
Pfizer Inc.
GlaxoSmithKline plc
Johnson & Johnson
Merck & Co.
Roche Holding AG
Sanofi S.A.
Novartis International AG
AbbVie Inc.
AstraZeneca plc
Bayer AG
Table of Contents
1. Global Herpangina Treatment Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (Incidence Rate of Herpangina, Mortality Rate, Treatment Adoption Rate)
1.4. Market Segmentation Overview
2. Global Herpangina Treatment Market Size (In USD Mn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis (Based on Cases Treated, Drug Sales)
2.3. Key Market Developments and Milestones (Drug Approvals, Medical Guidelines Updates)
3. Global Herpangina Treatment Market Analysis
3.1. Growth Drivers
3.1.1. Rising Pediatric Population
3.1.2. Increasing Awareness of Viral Infections
3.1.3. Improvements in Diagnostic Technologies
3.1.4. Government and NGO Initiatives for Pediatric Health
3.2. Market Challenges
3.2.1. Limited Diagnostic Facilities in Rural Areas
3.2.2. Lack of Effective Antiviral Treatments
3.2.3. Resistance to Antiviral Medications
3.3. Opportunities
3.3.1. Development of Novel Antiviral Therapies
3.3.2. Rising Telemedicine Adoption in Developing Markets
3.3.3. Increasing Healthcare Investments in Low- and Middle-Income Countries (LMICs)
3.4. Trends
3.4.1. Increasing Use of Herbal Remedies and Natural Treatments
3.4.2. Emergence of Pediatric-focused Viral Treatment Centers
3.4.3. Increasing Research on Vaccine Development
3.5. Government Regulations
3.5.1. WHO Guidelines on Pediatric Viral Infections
3.5.2. National Health Program Interventions for Child Health
3.5.3. Mandatory Reporting of Viral Infections in Various Countries
3.6. SWOT Analysis
3.7. Stake Ecosystem (Hospitals, Pediatricians, Pharmacists, Drug Manufacturers, NGOs)
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. Global Herpangina Treatment Market Segmentation
4.1. By Treatment Type (In Value %)
4.1.1. Antiviral Medications
4.1.2. Pain Relievers
4.1.3. Herbal Remedies
4.1.4. Other Supportive Treatments
4.2. By Patient Age Group (In Value %)
4.2.1. Pediatric (0-5 years)
4.2.2. Pediatric (6-12 years)
4.2.3. Pediatric (13-18 years)
4.3. By Distribution Channel (In Value %)
4.3.1. Hospitals
4.3.2. Retail Pharmacies
4.3.3. Online Pharmacies
4.3.4. Drug Stores
4.4. By Diagnosis Method (In Value %)
4.4.1. Clinical Examination
4.4.2. Lab Testing (PCR, Viral Cultures)
4.5. By Region (In Value %)
4.5.1. North America
4.5.2. Europe
4.5.3. Asia Pacific
4.5.4. Latin America
4.5.5. Middle East & Africa
5. Global Herpangina Treatment Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Pfizer Inc.
5.1.2. GlaxoSmithKline plc
5.1.3. Johnson & Johnson
5.1.4. Merck & Co.
5.1.5. Roche Holding AG
5.1.6. Sanofi S.A.
5.1.7. Novartis International AG
5.1.8. AbbVie Inc.
5.1.9. AstraZeneca plc
5.1.10. Bayer AG
5.1.11. Gilead Sciences Inc.
5.1.12. Takeda Pharmaceutical Company Limited
5.1.13. Cipla Ltd.
5.1.14. Dr. Reddys Laboratories
5.1.15. Teva Pharmaceutical Industries Ltd.
5.2. Cross Comparison Parameters (Revenue from Pediatric Drugs, Antiviral Research Investment, Pediatric Drug Pipelines, R&D Spending, Patent Portfolio, Global Reach, Market Share in Antiviral Medications, Number of Clinical Trials)
5.3. Market Share Analysis
5.4. Strategic Initiatives (R&D, Product Launches, Clinical Trials)
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. Global Herpangina Treatment Market Regulatory Framework
6.1. Health and Safety Standards for Pediatric Medications
6.2. FDA and EMA Approvals for Herpangina Treatments
6.3. Compliance with WHO Guidelines
7. Global Herpangina Treatment Future Market Size (In USD Mn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. Global Herpangina Treatment Future Market Segmentation
8.1. By Treatment Type (In Value %)
8.2. By Patient Age Group (In Value %)
8.3. By Distribution Channel (In Value %)
8.4. By Diagnosis Method (In Value %)
8.5. By Region (In Value %)
9. Global Herpangina Treatment Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis (Pediatric Hospitals, Pharmacies, NGOs, Pediatric Clinics)
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis (Emerging Markets, Telemedicine, Pediatric Clinical Care)
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The initial phase involves constructing a comprehensive market map encompassing all key stakeholders in the global herpangina treatment market. Extensive secondary research was conducted using proprietary databases and government health statistics to identify critical variables such as patient demographics, incidence rates, and treatment options.
Step 2: Market Analysis and Construction
The second phase includes gathering historical data related to herpangina treatment sales, incidence rates, and healthcare provider data. This step involved analyzing global market penetration rates, revenue generated from pharmaceutical sales, and clinical trial data to form a detailed view of market trends and opportunities.
Step 3: Hypothesis Validation and Expert Consultation
In this phase, market hypotheses were validated through in-depth consultations with key opinion leaders, including pediatricians, healthcare administrators, and pharmaceutical industry experts. Their insights helped to refine market projections and verify data accuracy.
Step 4: Research Synthesis and Final Output
The final phase involved synthesizing the data into a comprehensive market report. Direct feedback from healthcare providers and pharmaceutical companies was incorporated to provide a validated, thorough analysis of the global herpangina treatment market.
Frequently Asked Questions
01. How big is the Global Herpangina Treatment Market?
The global herpangina treatment market is valued at USD 1.65 billion, driven by rising incidences of viral infections among pediatric populations and increasing demand for effective antiviral treatments.
02. What are the challenges in the Global Herpangina Treatment Market?
Challenges include limited availability of diagnostic facilities in rural areas, lack of effective antiviral medications, and increasing resistance to existing antiviral treatments, which hinder market growth.
03. Who are the major players in the Global Herpangina Treatment Market?
Major players in the market include Pfizer Inc., GlaxoSmithKline plc, Johnson & Johnson, Merck & Co., and Roche Holding AG. These companies dominate due to their extensive research and development efforts and established distribution networks.
04. What are the growth drivers of the Global Herpangina Treatment Market?
Key drivers include increasing incidences of viral infections, growing awareness of pediatric healthcare, and advancements in diagnostic technologies, particularly in developed regions with strong healthcare infrastructures.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















