Region:Middle East
Author(s):Shubham
Product Code:KRAD5367
Pages:99
Published On:December 2025
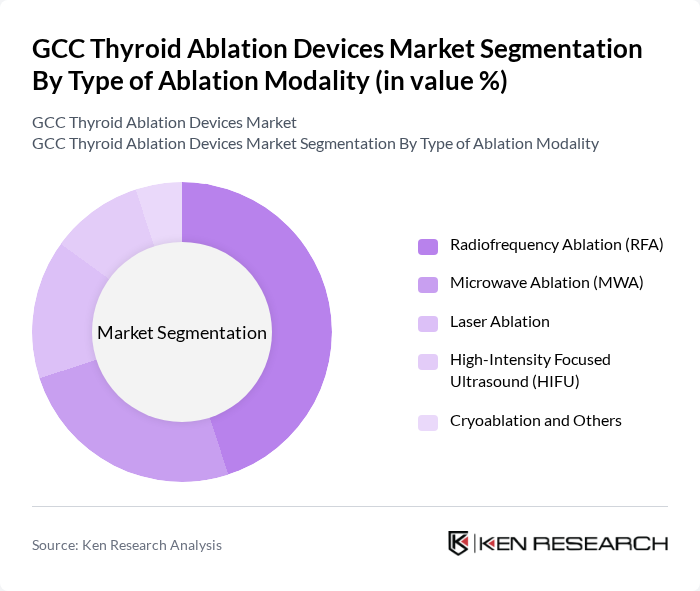
By Type of Ablation Modality:This segmentation includes various techniques used for thyroid ablation, which are essential for treating thyroid conditions effectively. The subsegments are Radiofrequency Ablation (RFA), Microwave Ablation (MWA), Laser Ablation, High-Intensity Focused Ultrasound (HIFU), and Cryoablation and Others. Among these, Radiofrequency Ablation (RFA) is the most widely adopted method for benign thyroid nodules and selected low?risk cancers, due to its strong clinical evidence, effectiveness, minimal invasiveness, and favorable patient outcomes. Global analyses indicate that radiofrequency ablation devices account for the largest share among thyroid ablation modalities, with microwave ablation gaining traction as an emerging alternative. The increasing preference for outpatient and day?surgery procedures, reduced recovery times compared with open surgery, and the growing number of interventional radiologists and endocrine surgeons trained in these techniques further solidify RFA’s dominance in the market.
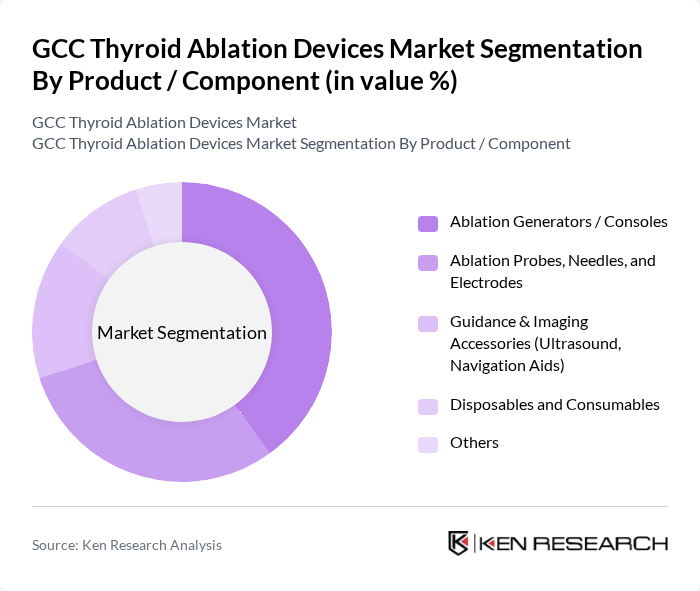
By Product / Component:This segmentation focuses on the various products and components used in thyroid ablation procedures. The subsegments include Ablation Generators / Consoles, Ablation Probes, Needles, and Electrodes, Guidance & Imaging Accessories (Ultrasound, Navigation Aids), Disposables and Consumables, and Others. The Ablation Generators / Consoles segment leads the market due to their critical role in the ablation process, providing the necessary energy delivery, treatment algorithms, and control for effective and reproducible treatment. Increasing adoption of advanced radiofrequency and microwave platforms with integrated temperature monitoring, impedance tracking, and user?friendly interfaces, along with the need for precision in image?guided procedures, further enhances the demand for these devices.
The GCC Thyroid Ablation Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Boston Scientific Corporation, Johnson & Johnson (Ethicon), Terumo Corporation, Olympus Corporation, Integra LifeSciences Corporation, StarMed Co., Ltd., MedWaves, Inc., BVM Medical System Ltd., RGS Healthcare, Cambridge Interventional, LLC, Baird Medical Devices Co., Ltd., RFAMD, Inc., Elesta S.p.A., Theraclion SA contribute to innovation, geographic expansion, and service delivery in this space.
The future of the GCC thyroid ablation devices market appears promising, driven by technological advancements and increasing healthcare investments. As the region's healthcare infrastructure expands, more facilities will adopt innovative ablation technologies. Additionally, the rising geriatric population, projected to reach 9 million in future, will further fuel demand for effective thyroid treatments. The focus on outpatient procedures will also enhance patient access, making thyroid ablation a preferred choice for many.
| Segment | Sub-Segments |
|---|---|
| By Type of Ablation Modality | Radiofrequency Ablation (RFA) Microwave Ablation (MWA) Laser Ablation High-Intensity Focused Ultrasound (HIFU) Cryoablation and Others |
| By Product / Component | Ablation Generators / Consoles Ablation Probes, Needles, and Electrodes Guidance & Imaging Accessories (Ultrasound, Navigation Aids) Disposables and Consumables Others |
| By Application | Benign Thyroid Nodules Recurrent or Residual Thyroid Cancer Primary Malignant Thyroid Tumors Hyperfunctioning / Autonomously Functioning Thyroid Nodules Others |
| By End-User | Tertiary Care Hospitals Specialty Endocrinology & Thyroid Clinics Ambulatory Surgical Centers (ASCs) Academic & Research Institutions Others |
| By Usage Type | Single-use / Disposable Devices Reusable Devices Reusable Generators with Disposable Probes |
| By Distribution Channel | Direct Sales to Hospitals and Clinics Regional Distributors / Importers Group Purchasing Organizations (GPOs) and Tender-Based Procurement Online and E-procurement Platforms Others |
| By GCC Country | Saudi Arabia United Arab Emirates Qatar Kuwait Oman Bahrain |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Endocrinologists in GCC Hospitals | 90 | Thyroid Specialists, Clinical Researchers |
| Medical Device Distributors | 70 | Sales Managers, Product Specialists |
| Healthcare Administrators | 60 | Procurement Officers, Hospital Managers |
| Patients with Thyroid Conditions | 80 | Thyroid Disease Patients, Support Group Leaders |
| Clinical Researchers in Thyroid Studies | 50 | Research Scientists, Academic Physicians |
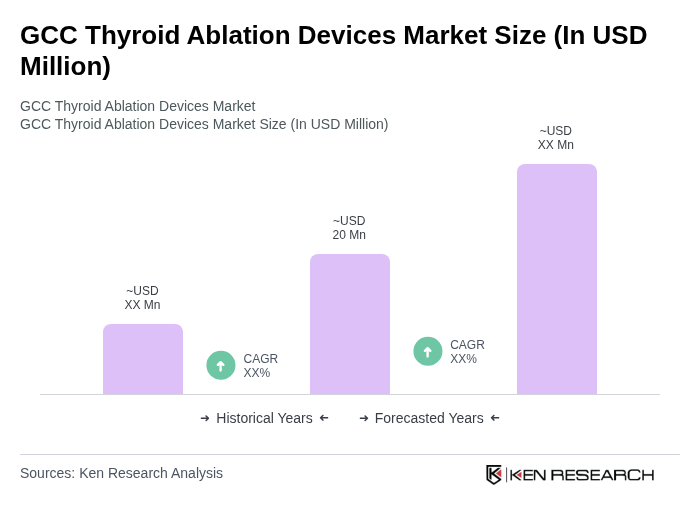
The GCC Thyroid Ablation Devices Market is valued at approximately USD 20 million, based on historical analysis and extrapolated regional shares from the global market, which is estimated to be between USD 170 million and USD 185 million.