Region:Global
Author(s):Rebecca
Product Code:KRAB0167
Pages:91
Published On:August 2025
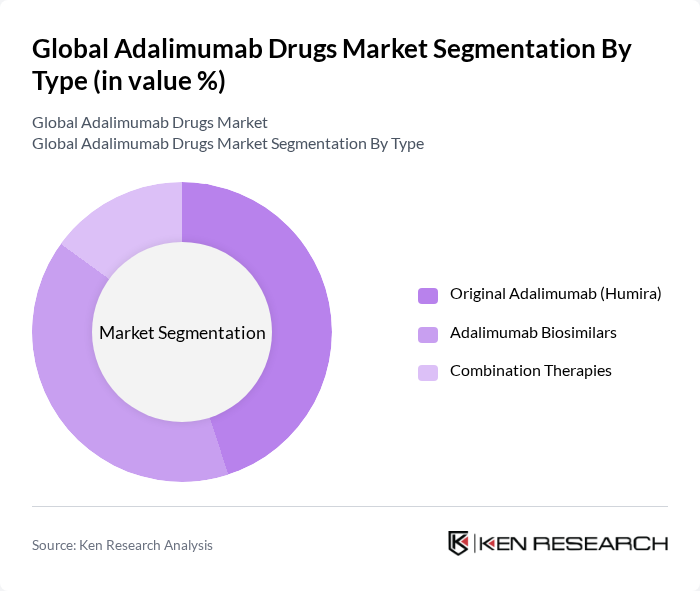
By Type:The market is segmented into three main types: Original Adalimumab (Humira), Adalimumab Biosimilars, and Combination Therapies. The Original Adalimumab remains a significant player due to its established efficacy and brand recognition. However, the rise of biosimilars has introduced competitive pricing and increased accessibility, attracting a broader patient demographic. Combination therapies are also gaining traction as they offer enhanced treatment outcomes for complex and refractory autoimmune conditions.
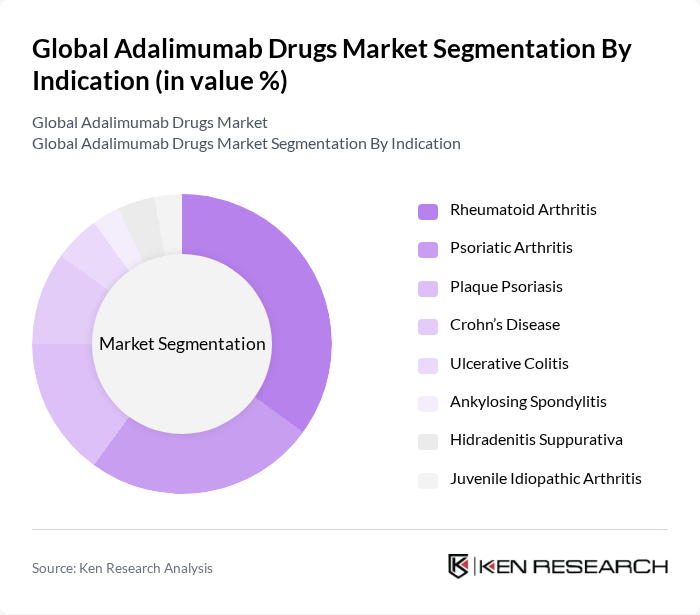
By Indication:The adalimumab market is primarily driven by its indications for various autoimmune diseases, including Rheumatoid Arthritis, Psoriatic Arthritis, and Plaque Psoriasis. Rheumatoid Arthritis remains the leading indication due to its high prevalence and the significant impact on patients' quality of life. Other indications such as Crohn’s Disease and Ulcerative Colitis are also notable contributors to overall demand, as the drug is widely prescribed for chronic inflammatory and immune-mediated conditions.
The Global Adalimumab Drugs Market is characterized by a dynamic mix of regional and international players. Leading participants such as AbbVie Inc., Amgen Inc., Pfizer Inc., Johnson & Johnson (Janssen Biotech, Inc.), Samsung Bioepis Co., Ltd., Novartis AG (Sandoz Division), Merck & Co., Inc. (MSD), Biogen Inc., Boehringer Ingelheim GmbH, Sandoz International GmbH, Mylan N.V. (now part of Viatris Inc.), Teva Pharmaceutical Industries Ltd., Fresenius Kabi AG, Celltrion Healthcare Co., Ltd., and Hetero Healthcare Limited contribute to innovation, geographic expansion, and service delivery in this space.
The future of the adalimumab market appears promising, driven by ongoing advancements in drug development and increasing patient awareness. As healthcare systems continue to evolve, the integration of personalized medicine and patient-centric approaches will likely enhance treatment efficacy. Additionally, the rise of telemedicine and digital health solutions is expected to facilitate better patient engagement and adherence to treatment regimens, ultimately improving health outcomes and expanding market reach for adalimumab therapies.
| Segment | Sub-Segments |
|---|---|
| By Type | Original Adalimumab (Humira) Adalimumab Biosimilars (e.g., Amjevita, Hyrimoz, Hadlima, Hulio, Idacio, Yuflyma, Abrilada, etc.) Combination Therapies (Adalimumab with other immunomodulators or DMARDs) |
| By Indication | Rheumatoid Arthritis Psoriatic Arthritis Plaque Psoriasis Crohn’s Disease Ulcerative Colitis Ankylosing Spondylitis Hidradenitis Suppurativa Juvenile Idiopathic Arthritis |
| By Administration Route | Subcutaneous Injection Intravenous Infusion (rare for adalimumab, but included for completeness) |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Online Pharmacies |
| By Patient Demographics | Pediatric Patients Adult Patients |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Rheumatology Clinics | 100 | Rheumatologists, Nurse Practitioners |
| Dermatology Practices | 80 | Dermatologists, Physician Assistants |
| Pharmacy Chains | 110 | Pharmacy Managers, Pharmacists |
| Patient Advocacy Groups | 50 | Patient Representatives, Healthcare Advocates |
| Healthcare Payers | 70 | Insurance Analysts, Medical Directors |
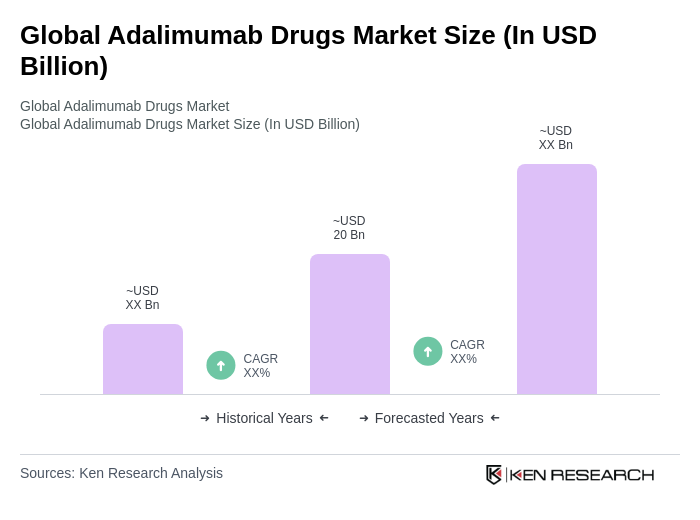
The Global Adalimumab Drugs Market is valued at approximately USD 20 billion, driven by the rising prevalence of autoimmune diseases, increased healthcare expenditure, and the introduction of cost-effective biosimilars, enhancing treatment accessibility for patients.