Region:Global
Author(s):Geetanshi
Product Code:KRAB0143
Pages:87
Published On:August 2025
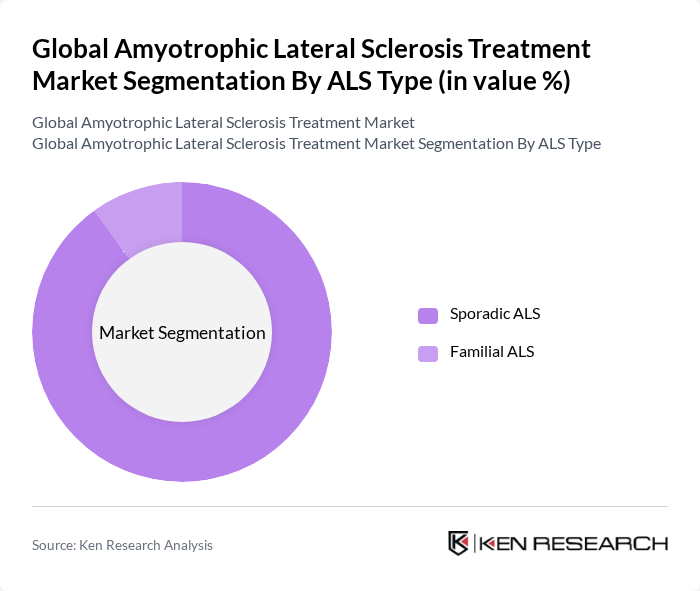
By ALS Type:The market is segmented into Sporadic ALS and Familial ALS. Sporadic ALS is the most common form, accounting for approximately 90% to 95% of cases, which drives its dominance in the market. Familial ALS, while less common, is significant due to its genetic implications and the targeted therapies being developed for it.
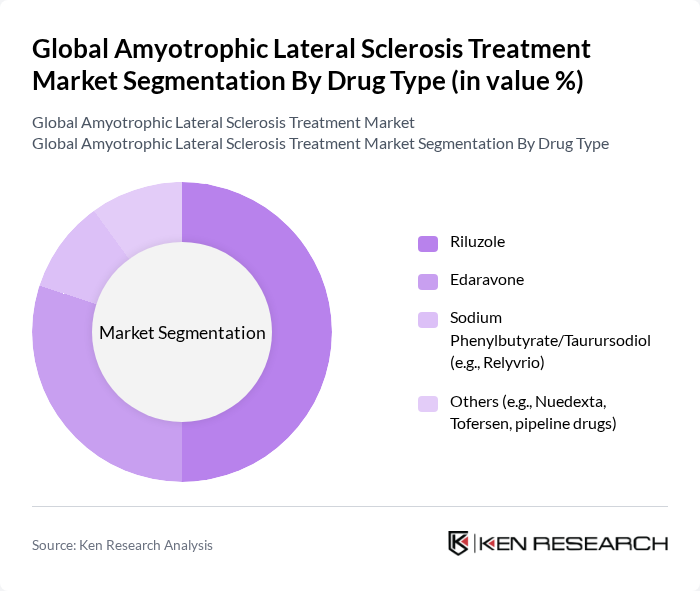
By Drug Type:The market is categorized into Riluzole, Edaravone, Sodium Phenylbutyrate/Taurursodiol (e.g., Relyvrio), and Others. Riluzole remains the leading drug due to its long-standing approval and established efficacy in prolonging survival. Edaravone continues to gain traction as a newer treatment option, while the "Others" category includes emerging therapies such as Nuedexta, Tofersen, and pipeline drugs currently in clinical trials.
The Global Amyotrophic Lateral Sclerosis Treatment Market is characterized by a dynamic mix of regional and international players. Leading participants such as Biogen Inc., Mitsubishi Tanabe Pharma Corporation, Amylyx Pharmaceuticals, Inc., Sanofi S.A., Teva Pharmaceutical Industries Ltd., Eli Lilly and Company, Merck & Co., Inc., F. Hoffmann-La Roche AG, Regeneron Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, Pfizer Inc., AbbVie Inc., Astellas Pharma Inc., GSK plc, Vertex Pharmaceuticals Incorporated contribute to innovation, geographic expansion, and service delivery in this space.
The future of the ALS treatment market appears promising, driven by ongoing research and technological advancements. The integration of artificial intelligence in treatment planning is expected to enhance personalized medicine approaches, improving patient outcomes. Additionally, the expansion of telemedicine services will facilitate better access to specialists, particularly in underserved regions. These trends indicate a shift towards more innovative and accessible treatment solutions, which will likely shape the market landscape in the coming years.
| Segment | Sub-Segments |
|---|---|
| By ALS Type | Sporadic ALS Familial ALS |
| By Drug Type | Riluzole Edaravone Sodium Phenylbutyrate/Taurursodiol (e.g., Relyvrio) Others (e.g., Nuedexta, Tofersen, pipeline drugs) |
| By Treatment Modality | Pharmaceutical Treatments Supportive Therapies (Physical, Occupational, Speech Therapy) Assistive Devices (Ventilators, Mobility Aids, Communication Devices) |
| By End-User | Hospitals Specialty Clinics Home Care Settings Others |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Online Pharmacies |
| By Region | North America Europe Asia Pacific Latin America Middle East & Africa |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Neurologists Specializing in ALS | 60 | Clinical Practitioners, Research Physicians |
| Pharmaceutical Executives in ALS Treatment | 45 | Product Managers, R&D Directors |
| Healthcare Policy Makers | 40 | Health Economists, Regulatory Affairs Specialists |
| Patient Advocacy Group Representatives | 50 | Advocacy Leaders, Community Outreach Coordinators |
| Clinical Trial Coordinators | 55 | Clinical Research Associates, Trial Managers |
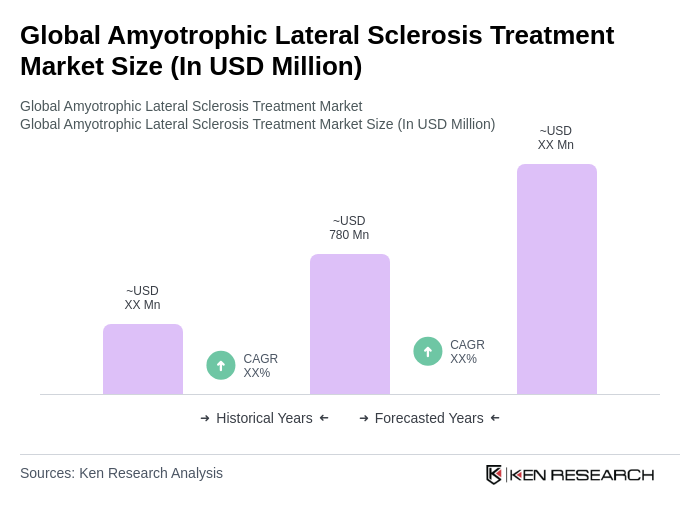
The Global Amyotrophic Lateral Sclerosis (ALS) Treatment Market is valued at approximately USD 780 million, reflecting a five-year historical analysis. This growth is driven by the increasing prevalence of ALS and advancements in drug development.