Region:Global
Author(s):Shubham
Product Code:KRAA1751
Pages:98
Published On:August 2025
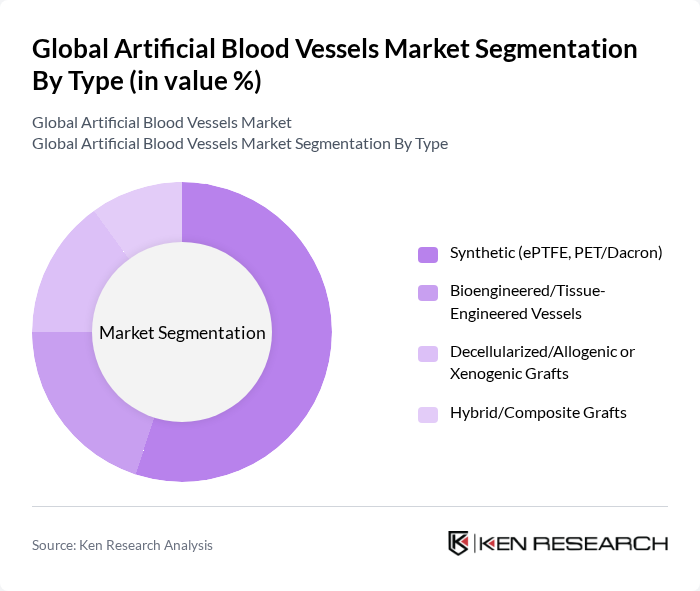
By Type:The market is segmented into various types of artificial blood vessels, including synthetic, bioengineered, decellularized, and hybrid grafts. Among these, synthetic grafts, particularly those made from expanded polytetrafluoroethylene (ePTFE) and polyethylene terephthalate (PET/Dacron), dominate the market due to their widespread use in surgical procedures and proven reliability. Bioengineered vessels are gaining traction due to advancements in tissue engineering, while decellularized grafts are preferred for their biocompatibility. Hybrid grafts are emerging as a versatile option, combining the benefits of synthetic and biological materials.
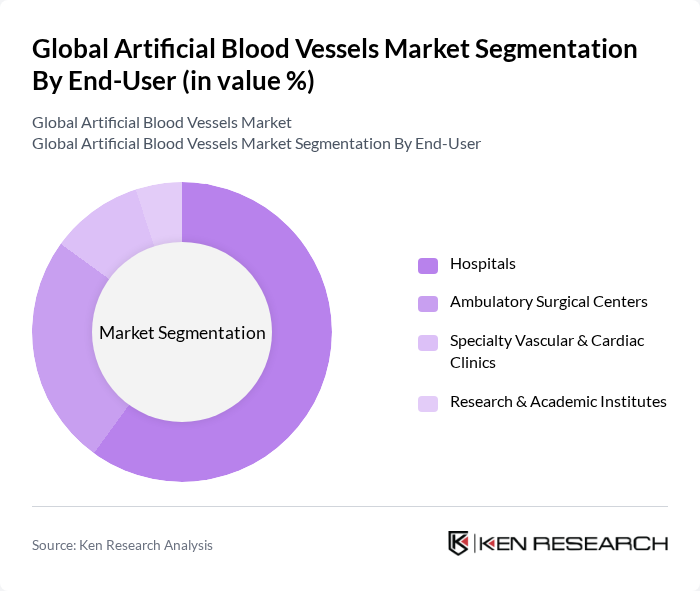
By End-User:The end-user segmentation includes hospitals, ambulatory surgical centers, specialty vascular and cardiac clinics, and research and academic institutes. Hospitals are the leading end-users, driven by the high volume of surgical procedures performed and the availability of advanced medical technologies. Ambulatory surgical centers are also witnessing growth due to the increasing preference for outpatient procedures. Specialty clinics are focusing on niche markets, while research institutes are pivotal in developing innovative graft technologies.
The Global Artificial Blood Vessels Market is characterized by a dynamic mix of regional and international players. Leading participants such as W. L. Gore & Associates, Inc. (Gore Medical), Terumo Corporation, B. Braun Melsungen AG, Cook Medical LLC, Getinge AB (Maquet Cardiovascular), LeMaitre Vascular, Inc., JOTEC GmbH (CryoLife/Artivion, Inc.), Medtronic plc, BD (Becton, Dickinson and Company), Humacyte, Inc., Suokang Medical (Lifetech Scientific Subsidiary), Shanghai Suokang Biomedical Co., Ltd., Xeltis B.V., CorMatrix Cardiovascular, Inc., Cardiva (part of Stockholm-headquartered Getinge) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the artificial blood vessels market in future appears promising, driven by technological advancements and increasing healthcare investments. As the demand for personalized medicine grows, manufacturers are likely to focus on developing tailored solutions that meet specific patient needs. Additionally, the integration of digital health technologies will enhance monitoring and management of cardiovascular conditions, further propelling market growth. The emphasis on sustainability will also shape product development, aligning with global trends toward eco-friendly healthcare solutions.
| Segment | Sub-Segments |
|---|---|
| By Type | Synthetic (ePTFE, PET/Dacron) Bioengineered/Tissue-Engineered Vessels Decellularized/Allogenic or Xenogenic Grafts Hybrid/Composite Grafts |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Vascular & Cardiac Clinics Research & Academic Institutes |
| By Application | Coronary Artery Bypass Grafting (CABG) Peripheral Artery Bypass (PAD/PVD) Hemodialysis Vascular Access (AV Grafts) Aortic & Endovascular Repair |
| By Material | Expanded Polytetrafluoroethylene (ePTFE) Polyethylene Terephthalate (PET/Dacron) Polyurethane & Other Polymers Biological Matrices (Collagen, Decellularized ECM) |
| By Distribution Channel | Direct Sales to Providers Distributors/Group Purchasing E-Procurement/Online Tenders Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Price Range | Low Price Medium Price High Price Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiovascular Surgery Departments | 120 | Cardiologists, Vascular Surgeons |
| Medical Device Distributors | 100 | Sales Managers, Product Specialists |
| Hospital Procurement Teams | 80 | Procurement Officers, Supply Chain Managers |
| Research & Development Units | 70 | R&D Managers, Biomedical Engineers |
| Regulatory Affairs Experts | 60 | Regulatory Affairs Managers, Compliance Officers |
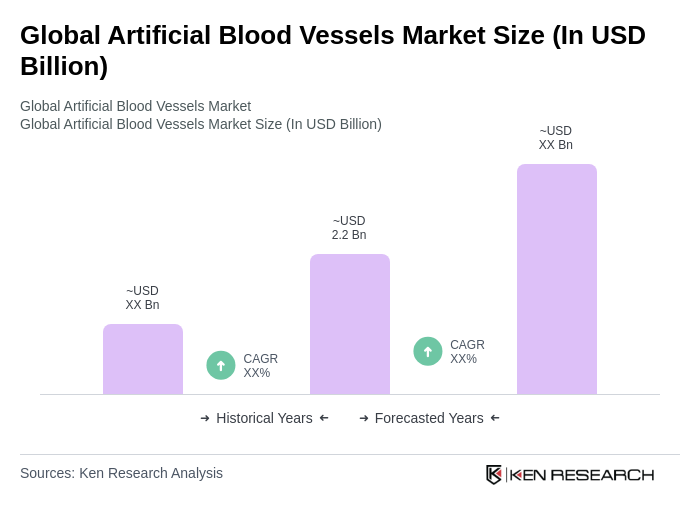
The Global Artificial Blood Vessels Market is valued at approximately USD 2.2 billion, with estimates ranging between USD 2.1 billion and USD 2.3 billion. This valuation reflects a five-year historical analysis and recent industry assessments.