Region:Global
Author(s):Shubham
Product Code:KRAA1873
Pages:95
Published On:August 2025
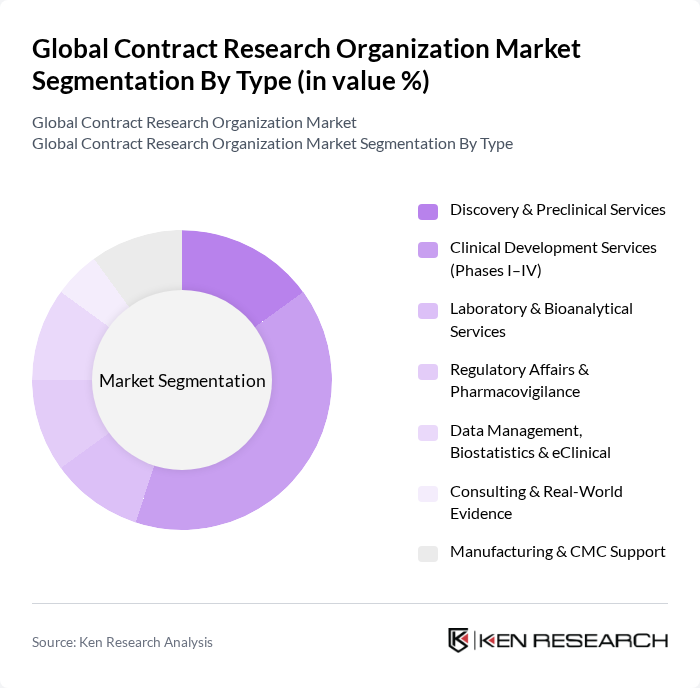
By Type:The market is segmented into various types of services offered by CROs. The primary subsegments include Discovery & Preclinical Services, Clinical Development Services (Phases I–IV), Laboratory & Bioanalytical Services, Regulatory Affairs & Pharmacovigilance, Data Management, Biostatistics & eClinical, Consulting & Real-World Evidence, and Manufacturing & CMC Support. Among these, Clinical Development Services (Phases I–IV) is the leading subsegment, driven by the increasing complexity of clinical trials and the need for specialized expertise in managing various phases of drug development. Recent trends reinforcing this leadership include decentralized and hybrid trial models, broader adoption of eClinical platforms, and risk-based monitoring to enhance data quality and trial efficiency .
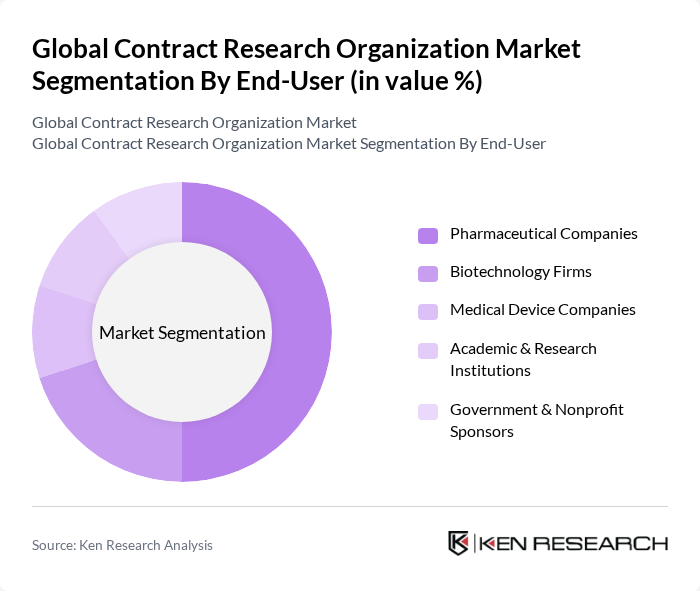
By End-User:The end-user segmentation includes Pharmaceutical Companies, Biotechnology Firms, Medical Device Companies, Academic & Research Institutions, and Government & Nonprofit Sponsors. Pharmaceutical Companies represent the largest share of the market, as they heavily rely on CROs for clinical trials and regulatory support, allowing them to focus on core competencies while reducing operational costs. Industry reporting consistently shows pharma and biopharma sponsors as the dominant client group for CRO services .
The Global Contract Research Organization Market is characterized by a dynamic mix of regional and international players. Leading participants such as IQVIA Holdings Inc., Labcorp Drug Development (formerly Covance), Parexel International Corporation, Charles River Laboratories International, Inc., PPD, Inc. (Thermo Fisher Scientific), Syneos Health, Inc., Medpace Holdings, Inc., WuXi AppTec Co., Ltd., ICON plc (including PRA Health Sciences), KCR S.A., dMed-Clinipace (now part of Simtra BioPharma Solutions), Clario (formerly Bioclinica), Worldwide Clinical Trials, Ergomed plc, Celerion contribute to innovation, geographic expansion, and service delivery in this space .
The future of the Contract Research Organization market appears promising, driven by technological advancements and evolving industry needs. The integration of artificial intelligence and machine learning is expected to enhance data analysis and streamline clinical trial processes. Additionally, the shift towards decentralized clinical trials will likely improve patient recruitment and retention, making trials more efficient. As the industry adapts to these trends, CROs will play a crucial role in facilitating innovation and ensuring compliance in an increasingly complex regulatory environment.
| Segment | Sub-Segments |
|---|---|
| By Type | Discovery & Preclinical Services Clinical Development Services (Phases I–IV) Laboratory & Bioanalytical Services Regulatory Affairs & Pharmacovigilance Data Management, Biostatistics & eClinical Consulting & Real-World Evidence Manufacturing & CMC Support |
| By End-User | Pharmaceutical Companies Biotechnology Firms Medical Device Companies Academic & Research Institutions Government & Nonprofit Sponsors |
| By Service Model | Full-Service CROs Functional Service Provider (FSP) Hybrid/Embedded Models Tactical/Project-Based Outsourcing |
| By Therapeutic Area | Oncology Cardiovascular & Metabolic Neurology Infectious Diseases & Vaccines Immunology & Inflammation Rare Diseases Others |
| By Phase of Development | Phase I Phase II Phase III Phase IV (Post-Marketing) Preclinical |
| By Geography | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Pricing Model | Fixed Fee Time & Materials Milestone/Performance-Based Risk-Sharing/Outcome-Based |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Clinical Trial Management | 120 | Clinical Operations Managers, Project Managers |
| Regulatory Affairs Consulting | 100 | Regulatory Affairs Specialists, Compliance Officers |
| Biostatistics and Data Management | 80 | Biostatisticians, Data Managers |
| Pharmacovigilance Services | 70 | Pharmacovigilance Officers, Safety Managers |
| Market Access and Pricing Strategy | 90 | Market Access Managers, Pricing Analysts |
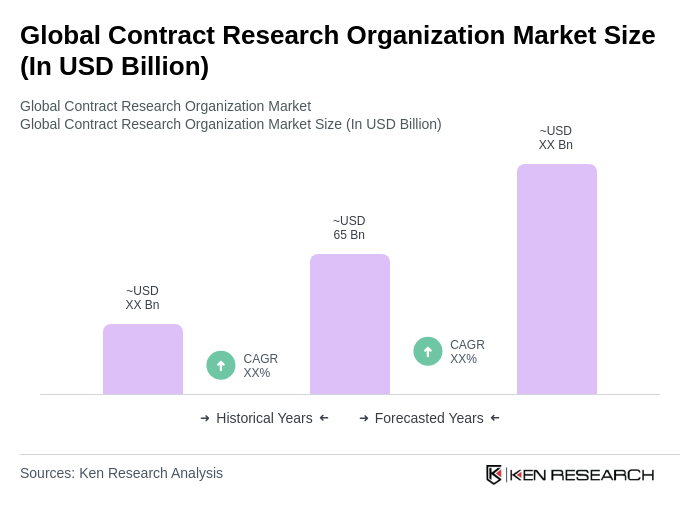
The Global Contract Research Organization Market is valued at approximately USD 65 billion, reflecting a sustained trend of outsourcing R&D and clinical operations to enhance speed, cost efficiency, and access to specialized capabilities.