Region:Global
Author(s):Shubham
Product Code:KRAD0770
Pages:94
Published On:August 2025

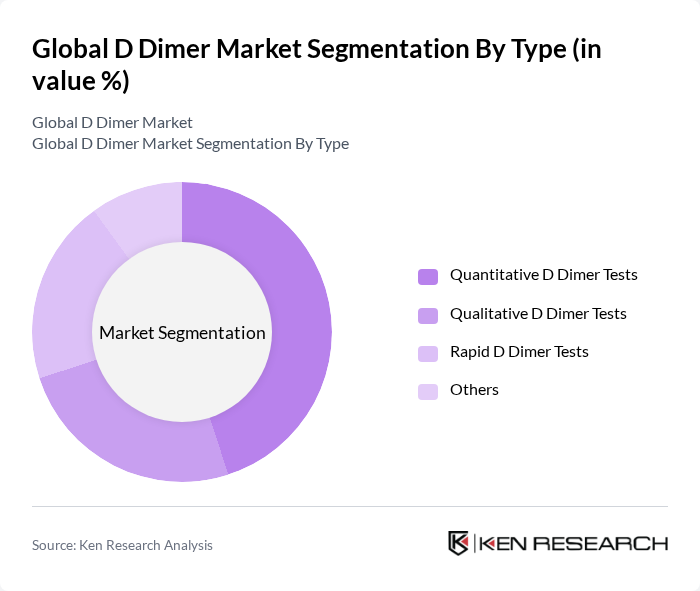
By Type:The D Dimer market is segmented into various types, including Quantitative D Dimer Tests, Qualitative D Dimer Tests, Rapid D Dimer Tests, and Others. Among these, Quantitative D Dimer Tests are leading the market due to their higher accuracy and reliability in diagnosing thromboembolic conditions. The increasing preference for precise diagnostic tools in clinical settings drives the demand for these tests, as they provide measurable results that aid in clinical decision-making.
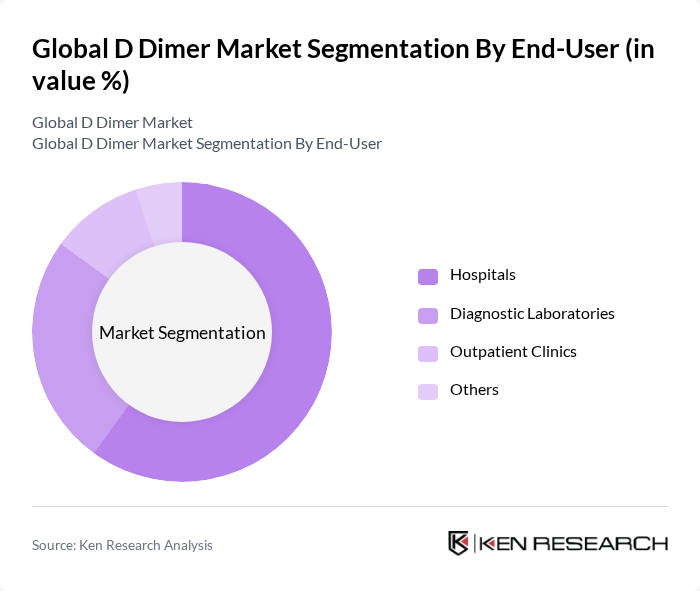
By End-User:The market is categorized by end-users, including Hospitals, Diagnostic Laboratories, Outpatient Clinics, and Others. Hospitals are the dominant end-user segment, primarily due to their extensive patient base and the need for rapid diagnostic services. The increasing number of hospital admissions related to thromboembolic disorders further fuels the demand for D Dimer tests, as hospitals strive to enhance patient care and streamline diagnostic processes.
The Global D Dimer Market is characterized by a dynamic mix of regional and international players. Leading participants such as Siemens Healthineers, Roche Diagnostics (F. Hoffmann-La Roche Ltd.), Abbott Laboratories, Thermo Fisher Scientific, Bio-Rad Laboratories, QuidelOrtho Corporation, Beckman Coulter (Danaher), Sysmex Corporation, FUJIREBIO, Werfen, DiaSorin, Grifols, Mindray (Shenzhen Mindray Bio-Medical Electronics Co., Ltd.), Sekisui Diagnostics, Randox Laboratories, HORIBA, Ltd., Diazyme Laboratories, BioMedica Diagnostics, Getein Biotech, Inc., LumiraDx, Helena Laboratories, CTK Biotech, Accurex Biomedical, Laboratory Corporation of America Holdings (Labcorp) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the D Dimer market is poised for significant transformation, driven by technological advancements and evolving healthcare paradigms. The integration of artificial intelligence in diagnostic processes is expected to enhance accuracy and efficiency, while the shift towards personalized medicine will tailor testing approaches to individual patient needs. As telemedicine continues to expand, remote diagnostics will facilitate greater access to D Dimer testing, particularly in underserved areas, ensuring timely interventions and improved patient care.
| Segment | Sub-Segments |
|---|---|
| By Type | Quantitative D Dimer Tests Qualitative D Dimer Tests Rapid D Dimer Tests Others |
| By End-User | Hospitals Diagnostic Laboratories Outpatient Clinics Others |
| By Application | Pulmonary Embolism Diagnosis Deep Vein Thrombosis Diagnosis Disseminated Intravascular Coagulation (DIC) Stroke and Other Thromboembolic Conditions |
| By Distribution Channel | Direct Sales Online Sales Distributors Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Pricing Model | Premium Pricing Competitive Pricing Value-Based Pricing Others |
| By Test Format | Laboratory-Based Tests Point-of-Care Tests Home Testing Kits Others |
| By Product | Instruments/Analyzers Reagents and Kits Controls and Calibrators Accessories and Consumables |
| By Assay Method | Enzyme-Linked Immunosorbent Assay (ELISA) Latex Immunoturbidimetric Assay Fluorescence Immunoassay Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Laboratories | 150 | Laboratory Managers, Clinical Pathologists |
| Diagnostic Device Manufacturers | 100 | Product Development Managers, Sales Directors |
| Healthcare Procurement Departments | 120 | Procurement Officers, Supply Chain Managers |
| Clinical Research Organizations | 80 | Clinical Research Coordinators, Data Analysts |
| Regulatory Bodies | 50 | Regulatory Affairs Specialists, Compliance Officers |
The Global D Dimer Market is valued at approximately USD 1.5 billion, driven by the increasing prevalence of thromboembolic disorders and advancements in diagnostic technologies. This market is expected to grow significantly in the coming years due to rising demand for accurate diagnostic tools.