Region:Global
Author(s):Rebecca
Product Code:KRAD0284
Pages:100
Published On:August 2025
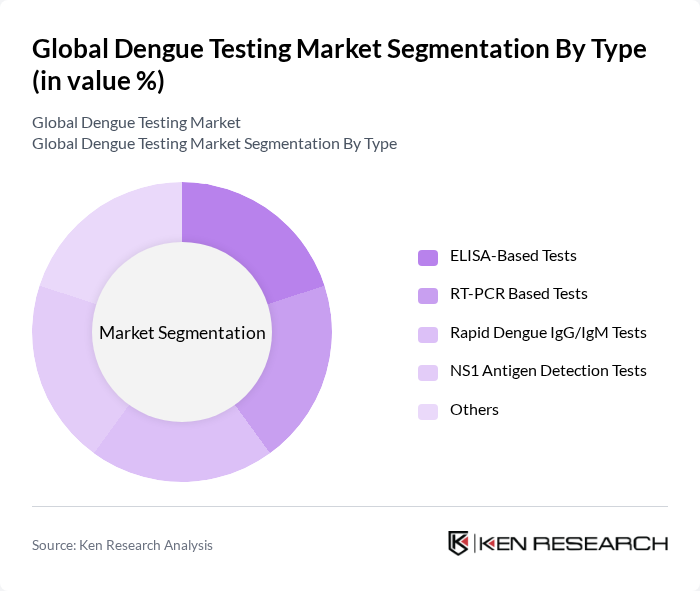
By Type:The market is segmented into various types of dengue testing methods, including ELISA-Based Tests, RT-PCR Based Tests, Rapid Dengue IgG/IgM Tests, NS1 Antigen Detection Tests, and Others. Among these, ELISA-Based Tests are currently the most widely used due to their reliability and cost-effectiveness. RT-PCR tests are gaining traction for their high sensitivity and specificity, particularly in clinical and research settings. The demand for rapid tests is also increasing, driven by the need for quick results in outbreak and point-of-care situations.
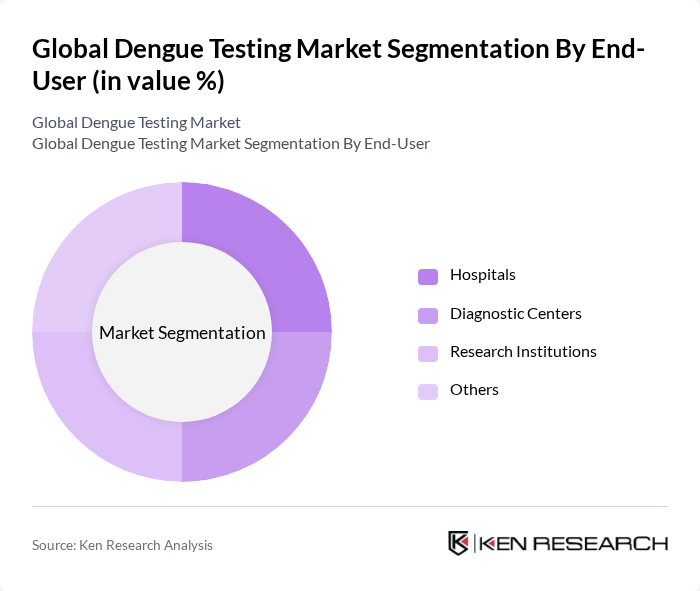
By End-User:The end-user segmentation includes Hospitals, Diagnostic Centers, Research Institutions, and Others. Diagnostic centers are significant end-users of dengue testing services, reflecting their specialized role in providing rapid and accurate diagnostics. Hospitals also remain key end-users, particularly for managing severe cases and supporting comprehensive care.
The Global Dengue Testing Market is characterized by a dynamic mix of regional and international players. Leading participants such as Abbott Laboratories, F. Hoffmann-La Roche AG, Thermo Fisher Scientific Inc., Bio-Rad Laboratories, Inc., Cepheid (a Danaher company), Siemens Healthineers AG, Becton, Dickinson and Company, QuidelOrtho Corporation, InBios International, Inc., NovaTec Immundiagnostica GmbH, CerTest Biotec S.L., DiaSorin S.p.A., Quest Diagnostics Incorporated, PerkinElmer Inc., Abnova Corporation, OriGene Technologies Inc., bioMérieux SA, MedMira Inc., Diagnostics for the Real World Ltd., and Mylab Discovery Solutions Pvt. Ltd. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the dengue testing market appears promising, driven by technological advancements and increased government focus on disease control. The integration of artificial intelligence in diagnostics is expected to enhance test accuracy and speed, while the shift towards point-of-care testing will facilitate quicker diagnosis in remote areas. Additionally, the growing emphasis on preventive healthcare will likely lead to increased investments in research and development, fostering innovation in dengue testing solutions.
| Segment | Sub-Segments |
|---|---|
| By Type | ELISA-Based Tests RT-PCR Based Tests Rapid Dengue IgG/IgM Tests NS1 Antigen Detection Tests Others |
| By End-User | Hospitals Diagnostic Centers Research Institutions Others |
| By Distribution Channel | Direct Sales Online Sales Distributors Others |
| By Region | North America Latin America Asia-Pacific Europe Middle East & Africa |
| By Application | Clinical Diagnosis Epidemiological Studies Blood Screening Others |
| By Sample Type | Blood Samples Serum Samples Plasma Samples Others |
| By Test Format | Rapid Test Kits Laboratory Tests Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Diagnostic Laboratories | 80 | Laboratory Managers, Technicians |
| Healthcare Providers | 70 | Infectious Disease Specialists, General Practitioners |
| Public Health Officials | 50 | Epidemiologists, Health Program Coordinators |
| Research Institutions | 40 | Research Scientists, Public Health Researchers |
| Government Health Agencies | 40 | Policy Makers, Health Administrators |
The Global Dengue Testing Market is valued at approximately USD 850 million, driven by the increasing incidence of dengue fever, heightened awareness of early diagnosis, and advancements in testing technologies.