Region:Global
Author(s):Rebecca
Product Code:KRAC0270
Pages:88
Published On:August 2025
By Therapy Type:The therapy type segmentation includes various treatment modalities aimed at managing Duchenne Muscular Dystrophy. The subsegments include Corticosteroids, Gene Therapy (e.g., Delandistrogene Moxeparvovec, Micro-dystrophin Therapies), Exon Skipping Therapies (e.g., Eteplirsen, Golodirsen, Viltolarsen, Casimersen), Antisense Oligonucleotides, Stem Cell Therapy, and Others (e.g., Utrophin Modulators, Anti-inflammatory Agents). Among these,Gene Therapy and molecular-based therapiesare currently dominating the market due to their innovative approach to addressing the underlying genetic causes of DMD, leading to improved patient outcomes and a growing acceptance among healthcare providers. Exon skipping therapies and corticosteroids remain widely used, while antisense oligonucleotides and stem cell therapies are emerging as promising options in clinical development .

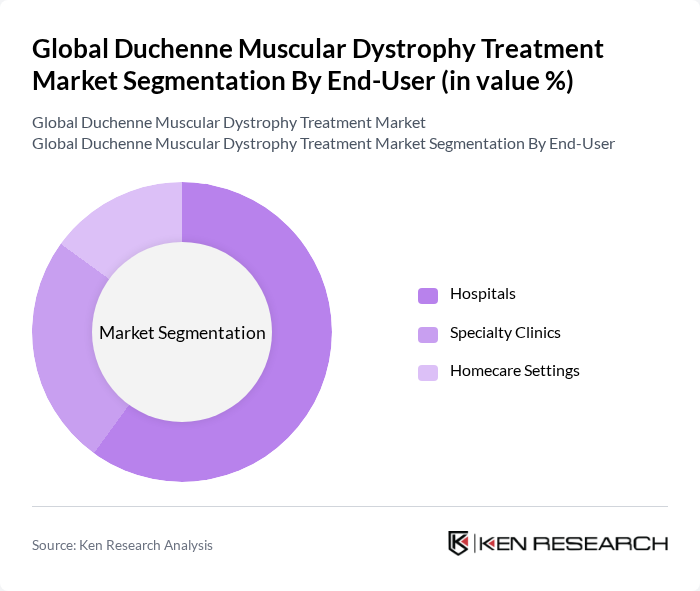
By End-User:The end-user segmentation encompasses the various settings where treatments for Duchenne Muscular Dystrophy are administered. This includes Hospitals, Specialty Clinics, and Homecare Settings.Hospitalsare the leading end-user segment, primarily due to their comprehensive facilities, access to specialized healthcare professionals, and ability to provide advanced treatment options and manage complex cases. Specialty clinics are increasingly important for outpatient care and clinical trial participation, while homecare settings are growing due to the availability of subcutaneous and oral therapies that improve patient convenience and adherence .
The Global Duchenne Muscular Dystrophy Treatment Market is characterized by a dynamic mix of regional and international players. Leading participants such as Sarepta Therapeutics, Inc., PTC Therapeutics, Inc., Pfizer Inc., Sanofi S.A., F. Hoffmann-La Roche AG, Novartis AG, Vertex Pharmaceuticals Incorporated, Solid Biosciences Inc., Astellas Pharma Inc., BioMarin Pharmaceutical Inc., Eli Lilly and Company, Amgen Inc., GlaxoSmithKline plc, Merck & Co., Inc., Takeda Pharmaceutical Company Limited, Dyne Therapeutics, Inc., Edgewise Therapeutics, Inc., Capricor Therapeutics, Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Duchenne Muscular Dystrophy treatment market appears promising, driven by ongoing advancements in gene therapy and a growing emphasis on personalized medicine. As the healthcare landscape evolves, the integration of digital health technologies is expected to enhance patient management and treatment adherence. Furthermore, increased collaboration between biotech firms and research institutions will likely accelerate the development of innovative therapies, ultimately improving patient outcomes and expanding market access.
| Segment | Sub-Segments |
|---|---|
| By Therapy Type | Corticosteroids Gene Therapy (e.g., Delandistrogene Moxeparvovec, Micro-dystrophin Therapies) Exon Skipping Therapies (e.g., Eteplirsen, Golodirsen, Viltolarsen, Casimersen) Antisense Oligonucleotides Stem Cell Therapy Others (e.g., Utrophin Modulators, Anti-inflammatory Agents) |
| By End-User | Hospitals Specialty Clinics Homecare Settings |
| By Route of Administration | Oral Intravenous Subcutaneous |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Online Pharmacies |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Patient Age Group | Pediatric Adolescent Adult |
| By Treatment Stage | Early Stage (Ambulatory) Mid Stage (Transitional) Late Stage (Non-ambulatory) |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Neurology Clinics | 100 | Neurologists, DMD Specialists |
| Pharmaceutical Companies | 70 | Product Managers, Clinical Research Directors |
| Patient Advocacy Groups | 50 | Advocacy Leaders, Caregivers |
| Healthcare Providers | 90 | General Practitioners, Pediatricians |
| Regulatory Bodies | 40 | Regulatory Affairs Specialists, Policy Makers |
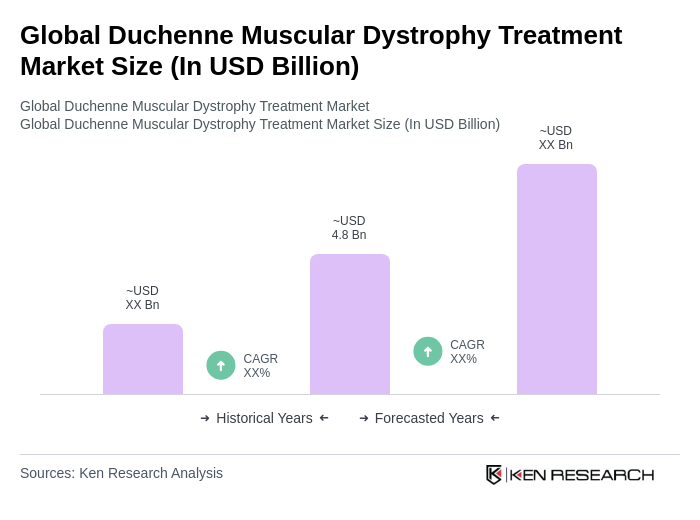
The Global Duchenne Muscular Dystrophy Treatment Market is valued at approximately USD 4.8 billion, driven by increasing awareness of genetic disorders, advancements in gene therapy, and the rising prevalence of Duchenne Muscular Dystrophy (DMD) worldwide.