Region:Global
Author(s):Geetanshi
Product Code:KRAC0162
Pages:96
Published On:August 2025
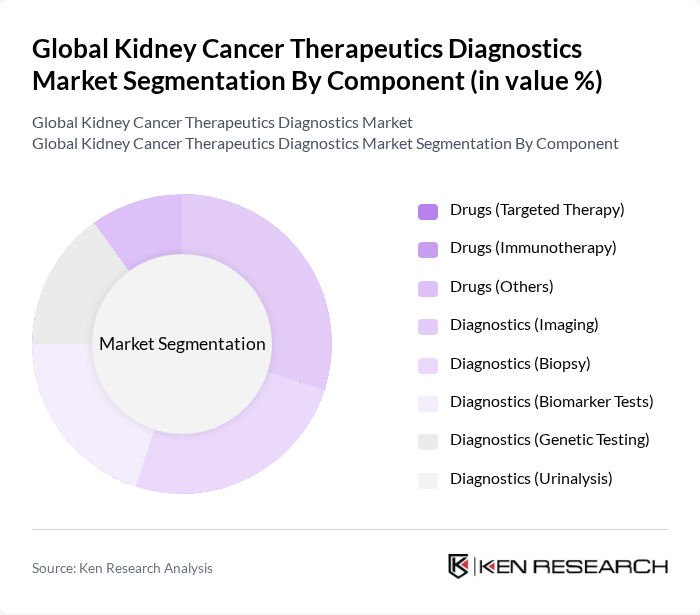
By Component:The market is segmented into two main components: Drugs and Diagnostics. The Drugs segment includes targeted therapy, immunotherapy, and other treatment options, while the Diagnostics segment encompasses imaging, biopsy, biomarker tests, genetic testing, and urinalysis. The Drugs segment is currently leading the market due to the increasing adoption of advanced therapies, such as immune checkpoint inhibitors and tyrosine kinase inhibitors, which offer improved patient outcomes and survival rates .
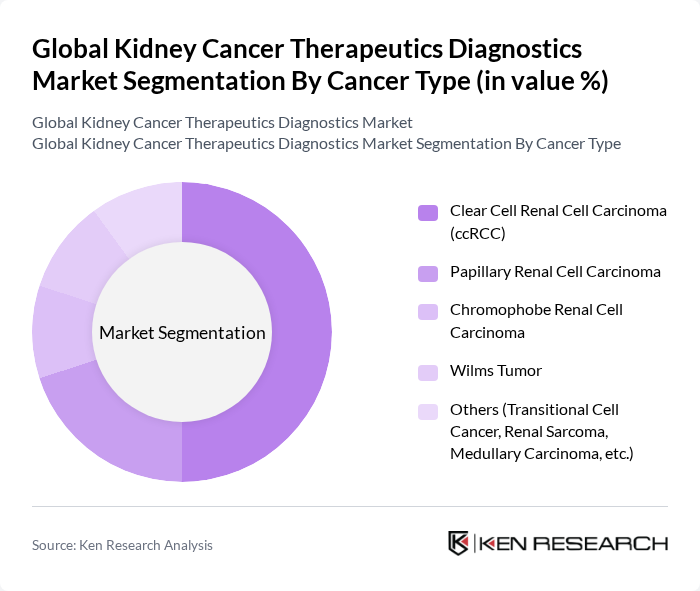
By Cancer Type:This segmentation includes Clear Cell Renal Cell Carcinoma (ccRCC), Papillary Renal Cell Carcinoma, Chromophobe Renal Cell Carcinoma, Wilms Tumor, and Others. The Clear Cell Renal Cell Carcinoma (ccRCC) segment is the most significant contributor to the market, driven by its high prevalence—accounting for the majority of kidney cancer cases—and the availability of targeted and immunotherapy options that have demonstrated improved clinical outcomes .
The Global Kidney Cancer Therapeutics Diagnostics Market is characterized by a dynamic mix of regional and international players. Leading participants such as F. Hoffmann-La Roche Ltd., Pfizer Inc., Novartis AG, Merck & Co., Inc., Bristol Myers Squibb, AstraZeneca plc, Exelixis, Inc., Eisai Co., Ltd., Bayer AG, AbbVie Inc., Siemens Healthineers, Abbott Laboratories, Thermo Fisher Scientific, Illumina, Inc., QIAGEN N.V., Agilent Technologies, Myriad Genetics, Inc., Hologic, Inc., Bio-Rad Laboratories, Inc., Exact Sciences Corporation, Becton, Dickinson and Company, Genomic Health, Inc. contribute to innovation, geographic expansion, and service delivery in this space .
The future of the kidney cancer therapeutics diagnostics market appears promising, driven by technological advancements and increasing healthcare investments. The integration of artificial intelligence in diagnostics is expected to enhance accuracy and efficiency, while personalized medicine will cater to individual patient needs. Additionally, the expansion of telemedicine services will improve access to care, particularly in underserved areas, fostering early detection and treatment. These trends indicate a robust growth trajectory for the market in future.
| Segment | Sub-Segments |
|---|---|
| By Component | Drugs (Targeted Therapy, Immunotherapy, Others) Diagnostics (Imaging, Biopsy, Biomarker Tests, Genetic Testing, Urinalysis) |
| By Cancer Type | Clear Cell Renal Cell Carcinoma (ccRCC) Papillary Renal Cell Carcinoma Chromophobe Renal Cell Carcinoma Wilms Tumor Others (Transitional Cell Cancer, Renal Sarcoma, Medullary Carcinoma, etc.) |
| By Application | Early Detection Disease Monitoring Treatment Decision Making Prognosis Assessment Others |
| By End-User | Hospitals Diagnostic Laboratories Research Institutions Home Care Settings |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Pricing Model | Premium Pricing Competitive Pricing Value-Based Pricing |
| By Technology | Molecular Diagnostics Imaging Technologies (CT, MRI, PET-CT, Ultrasound) Laboratory Developed Tests AI-enabled Diagnostics Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Oncologist Insights | 60 | Medical Oncologists, Urologists |
| Healthcare Provider Perspectives | 50 | Hospital Administrators, Clinical Pathologists |
| Patient Experience Feedback | 40 | Kidney Cancer Patients, Caregivers |
| Pharmaceutical Industry Insights | 40 | Product Managers, Market Access Specialists |
| Diagnostic Technology Providers | 40 | Diagnostic Lab Directors, Medical Device Manufacturers |
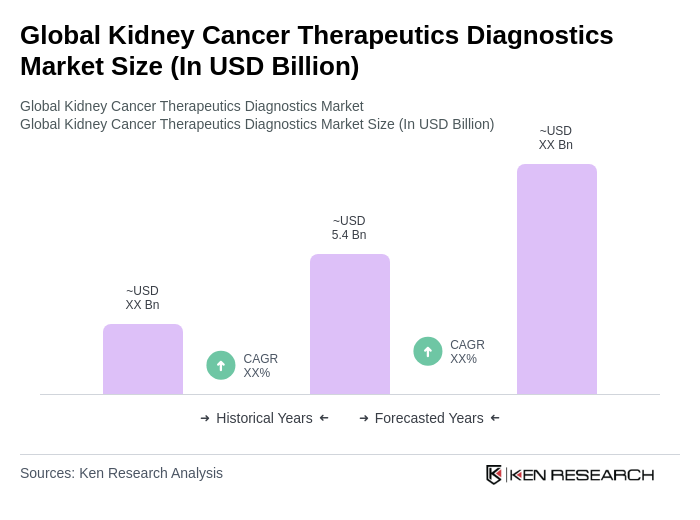
The Global Kidney Cancer Therapeutics Diagnostics Market is valued at approximately USD 5.4 billion, driven by the increasing prevalence of kidney cancer and advancements in diagnostic technologies, including artificial intelligence-based imaging and targeted therapies.