Region:Global
Author(s):Rebecca
Product Code:KRAD0322
Pages:91
Published On:August 2025
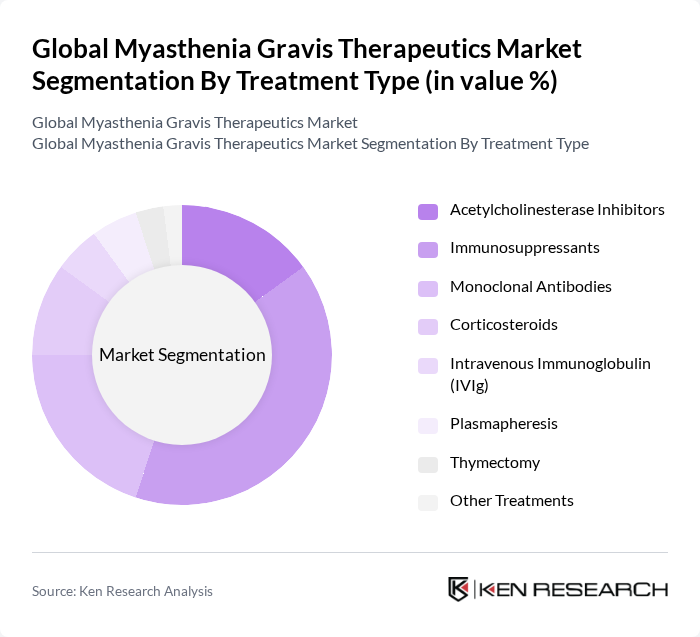
By Treatment Type:The treatment type segmentation includes various therapeutic approaches used to manage myasthenia gravis. The subsegments are Acetylcholinesterase Inhibitors, Immunosuppressants, Monoclonal Antibodies, Corticosteroids, Intravenous Immunoglobulin (IVIg), Plasmapheresis, Thymectomy, and Other Treatments. Among these, Immunosuppressants are currently dominating the market due to their effectiveness in reducing the immune response that contributes to the disease's symptoms. The increasing adoption of these therapies, along with the growing number of clinical trials and recent approvals of monoclonal antibodies, is driving their market share .
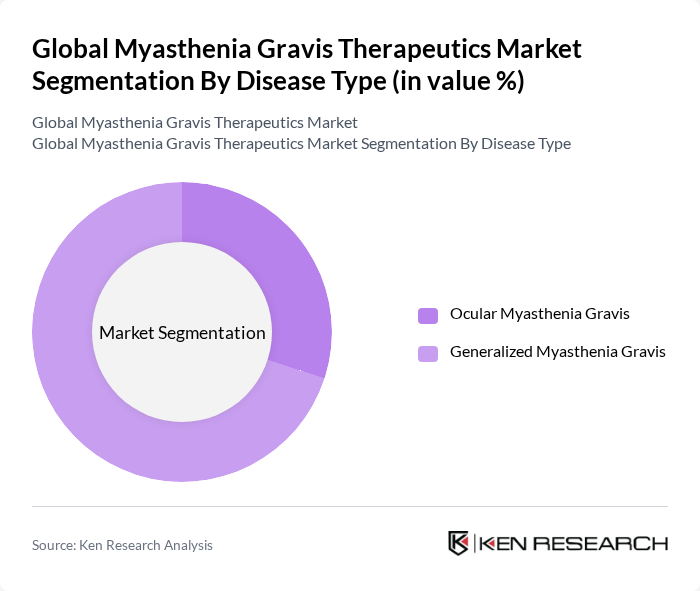
By Disease Type:The disease type segmentation includes Ocular Myasthenia Gravis and Generalized Myasthenia Gravis. Generalized Myasthenia Gravis is the leading subsegment, accounting for a significant portion of the market. This dominance is attributed to the higher prevalence of generalized forms of the disease compared to ocular forms, leading to increased demand for effective treatment options. The growing awareness and diagnosis of generalized myasthenia gravis further contribute to its market leadership. Generalized myasthenia gravis accounts for the majority of diagnosed cases and drives the largest share of therapeutic demand .
The Global Myasthenia Gravis Therapeutics Market is characterized by a dynamic mix of regional and international players. Leading participants such as AstraZeneca PLC, Novartis AG, Roche Holding AG, Sanofi S.A., Merck & Co., Inc., Pfizer Inc., Teva Pharmaceutical Industries Ltd., Amgen Inc., GSK plc (GlaxoSmithKline plc), UCB S.A., Eli Lilly and Company, Biogen Inc., AbbVie Inc., Johnson & Johnson, Astellas Pharma Inc., Alexion Pharmaceuticals, Inc. (AstraZeneca Rare Disease), Argenx SE, CSL Behring, Grifols, S.A., Takeda Pharmaceutical Company Limited contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Myasthenia Gravis therapeutics market is poised for significant transformation, driven by technological advancements and a shift towards personalized medicine. As the healthcare landscape evolves, the integration of telemedicine and digital health solutions is expected to enhance patient engagement and treatment adherence. Furthermore, ongoing collaborations between pharmaceutical companies and research institutions will likely accelerate the development of innovative therapies, ensuring that patients receive tailored treatment options that address their unique needs and improve overall health outcomes.
| Segment | Sub-Segments |
|---|---|
| By Treatment Type | Acetylcholinesterase Inhibitors Immunosuppressants Monoclonal Antibodies Corticosteroids Intravenous Immunoglobulin (IVIg) Plasmapheresis Thymectomy Other Treatments |
| By Disease Type | Ocular Myasthenia Gravis Generalized Myasthenia Gravis |
| By End-User | Hospitals Specialty Clinics Homecare Settings |
| By Route of Administration | Oral Intravenous Subcutaneous |
| By Patient Demographics | Adults Pediatric |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Online Pharmacies |
| By Treatment Duration | Short-term Long-term |
| By Pricing Tier | Premium Mid-range Economy |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Neurologists Specializing in Autoimmune Disorders | 80 | Neurologists, Clinical Researchers |
| Pharmaceutical Executives in Myasthenia Gravis | 60 | Product Managers, R&D Directors |
| Patients with Myasthenia Gravis | 120 | Patients, Caregivers |
| Healthcare Payers and Insurers | 50 | Policy Makers, Reimbursement Specialists |
| Clinical Trial Investigators | 40 | Principal Investigators, Clinical Coordinators |
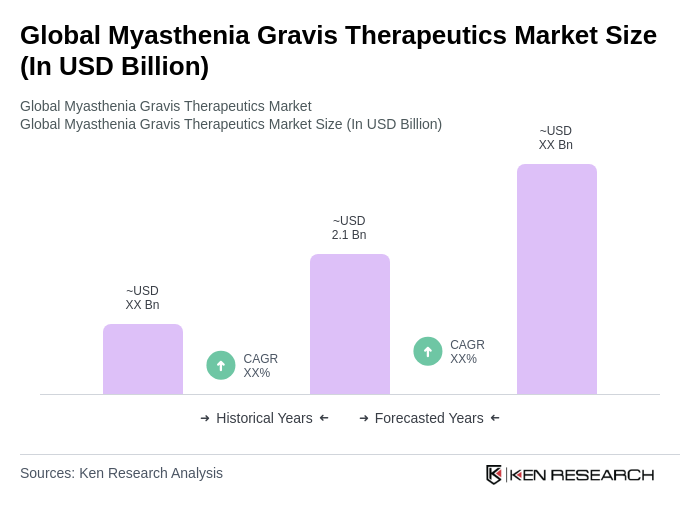
The Global Myasthenia Gravis Therapeutics Market is valued at approximately USD 2.1 billion, reflecting a significant growth driven by the increasing prevalence of the disease and advancements in treatment options such as monoclonal antibodies and immunosuppressants.