Region:Global
Author(s):Dev
Product Code:KRAD5148
Pages:84
Published On:December 2025
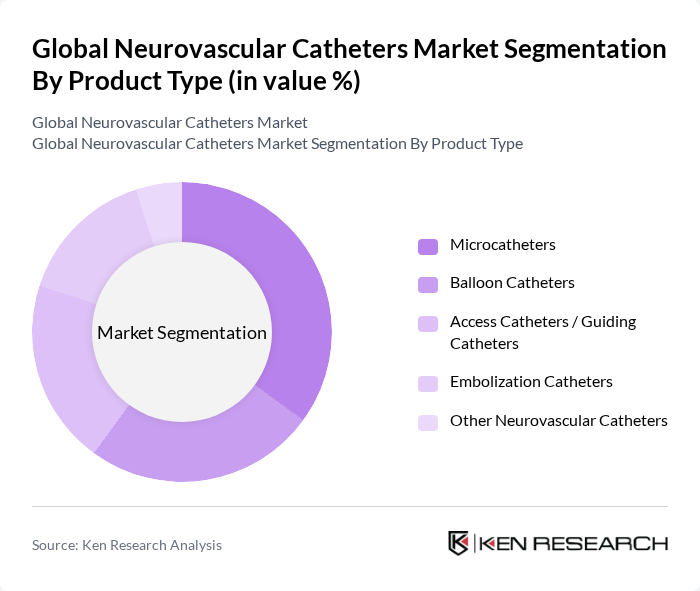
By Product Type:The product type segmentation includes various categories of neurovascular catheters, each serving specific medical needs. The subsegments are Microcatheters, Balloon Catheters, Access Catheters / Guiding Catheters, Embolization Catheters, and Other Neurovascular Catheters. Among these, Microcatheters are gaining traction due to their ability to navigate complex vascular structures, making them essential for intricate procedures.
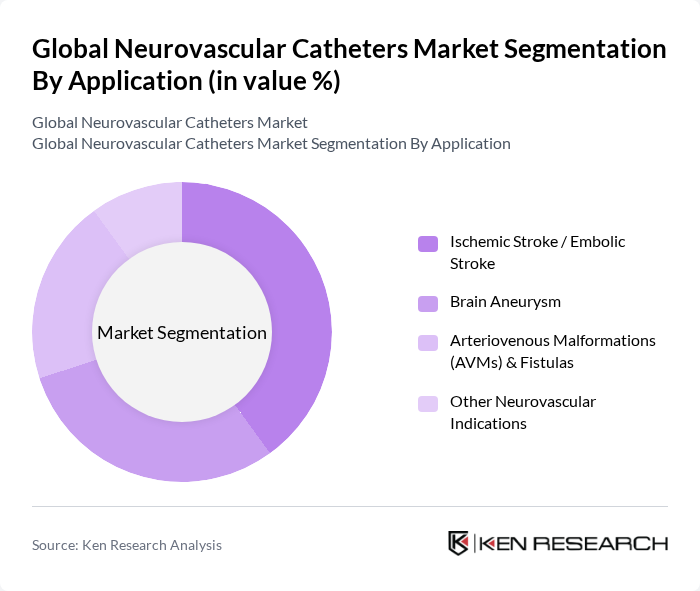
By Application:The application segmentation encompasses various medical conditions treated with neurovascular catheters, including Ischemic Stroke / Embolic Stroke, Brain Aneurysm, Arteriovenous Malformations (AVMs) & Fistulas, and Other Neurovascular Indications. The Ischemic Stroke / Embolic Stroke segment is currently the most significant due to the high incidence of strokes globally and the increasing awareness of timely intervention.
The Global Neurovascular Catheters Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Stryker Corporation, Penumbra, Inc., MicroVention, Inc. (a Terumo Group Company), Johnson & Johnson (Cerenovus), Boston Scientific Corporation, Terumo Corporation, Asahi Intecc Co., Ltd., Cook Medical LLC, Abbott Laboratories, Merit Medical Systems, Inc., B. Braun Melsungen AG, AngioDynamics, Inc., Balt Group (Balt Extrusion), Acandis GmbH contribute to innovation, geographic expansion, and service delivery in this space.
The future of the neurovascular catheters market appears promising, driven by ongoing technological advancements and an increasing focus on patient-centric care. As healthcare systems worldwide prioritize minimally invasive procedures, the demand for innovative catheter solutions is expected to rise. Additionally, the integration of digital technologies, such as telemedicine and remote monitoring, will enhance patient management and treatment outcomes, further propelling market growth in the coming years.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Microcatheters Balloon Catheters Access Catheters / Guiding Catheters Embolization Catheters Other Neurovascular Catheters |
| By Application | Ischemic Stroke / Embolic Stroke Brain Aneurysm Arteriovenous Malformations (AVMs) & Fistulas Other Neurovascular Indications |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Neurosurgery & Neurology Clinics Diagnostic & Imaging Centers |
| By Material | Polyurethane Silicone Polyethylene Other Polymers & Composite Materials |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Distribution Channel | Direct Sales to Hospitals & GPOs Third-Party Distributors / Dealers Online & E-Procurement Platforms Other Institutional Channels |
| By Pricing Tier | Premium-Branded Catheters Mid-Priced Catheters Economy / Value Catheters Contract / Tender-Based Pricing |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Neurosurgery Departments | 100 | Neurosurgeons, Surgical Coordinators |
| Interventional Radiology Units | 80 | Interventional Radiologists, Nurse Practitioners |
| Hospital Procurement Teams | 70 | Procurement Managers, Supply Chain Analysts |
| Medical Device Distributors | 60 | Sales Representatives, Product Managers |
| Clinical Research Organizations | 50 | Clinical Research Coordinators, Data Analysts |
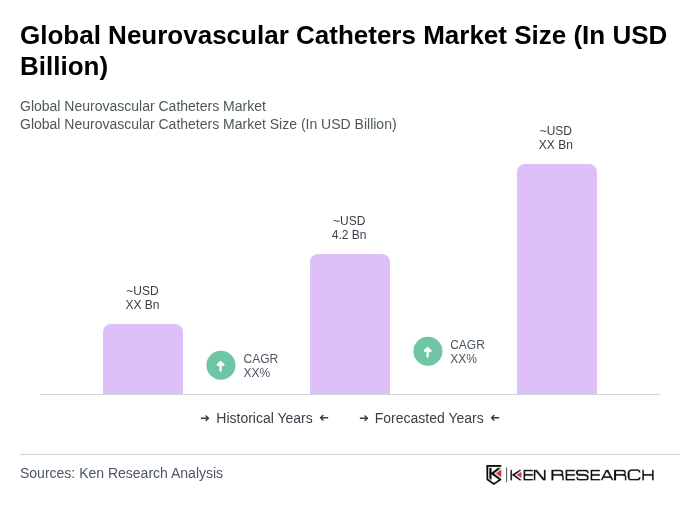
The Global Neurovascular Catheters Market is valued at approximately USD 4.2 billion, reflecting significant growth driven by the increasing prevalence of neurovascular diseases and advancements in catheter technology.