Region:Global
Author(s):Geetanshi
Product Code:KRAB0055
Pages:88
Published On:August 2025
By Therapy Type:The therapy type segmentation includes various treatment modalities used in managing pediatric neuroblastoma. The subsegments are Chemotherapy, Immunotherapy, Radiation Therapy, Surgery, Targeted Therapy, Stem Cell Transplant, and Others (e.g., Combination Therapies, Supportive Care). Each of these therapies plays a crucial role in the treatment landscape, with specific applications based on the patient's condition and stage of the disease.
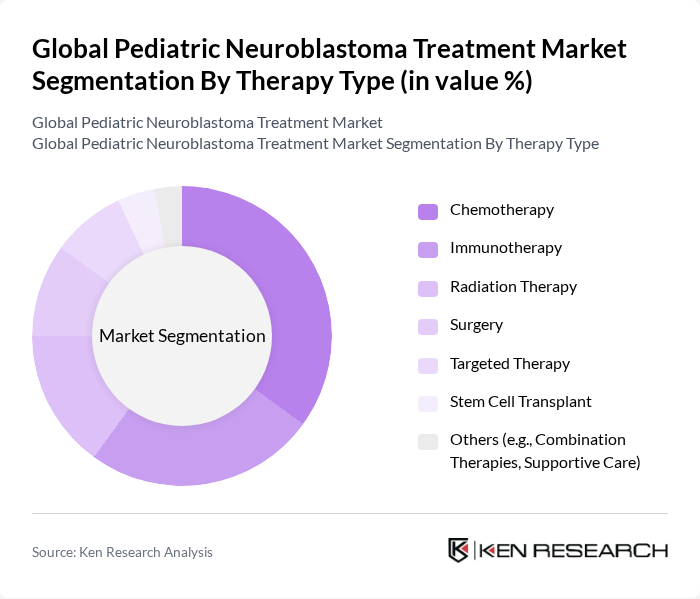
The chemotherapy subsegment dominates the market due to its established efficacy in treating neuroblastoma, particularly in its early stages. It is widely used as a first-line treatment and is often combined with other therapies to enhance overall effectiveness. The increasing number of clinical trials and research focused on optimizing chemotherapy regimens further supports its leading position. Immunotherapy is also gaining traction, particularly with recent advancements and regulatory approvals that have shown promising results in improving survival rates for high-risk pediatric neuroblastoma patients.
By Treatment Stage:The treatment stage segmentation categorizes the market based on the progression of neuroblastoma. The subsegments include Early Stage, Intermediate Stage, and Advanced/Relapsed Stage. Each stage requires different therapeutic approaches, influencing the choice of treatment and the overall market dynamics.

The early stage subsegment holds the largest market share as it encompasses a significant number of diagnosed cases that are amenable to curative treatment. The focus on early detection and intervention has led to improved outcomes, making this stage a priority for healthcare providers. The intermediate and advanced stages also represent critical areas of treatment, with ongoing research aimed at developing more effective therapies for relapsed cases.
The Global Pediatric Neuroblastoma Treatment Market is characterized by a dynamic mix of regional and international players. Leading participants such as Johnson & Johnson, Novartis AG, Merck & Co., Inc., Bristol-Myers Squibb Company, Amgen Inc., Eli Lilly and Company, Bayer AG, F. Hoffmann-La Roche AG, Takeda Pharmaceutical Company Limited, Sanofi S.A., AstraZeneca PLC, GSK plc (GlaxoSmithKline), Pfizer Inc., United Therapeutics Corporation, Y-mAbs Therapeutics, Inc., Blueprint Medicines Corporation, AbbVie Inc. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the pediatric neuroblastoma treatment market appears promising, driven by ongoing advancements in personalized medicine and the integration of technology in treatment protocols. As research continues to unveil new therapeutic targets, the development of targeted therapies is expected to gain momentum. Additionally, the expansion of clinical trials will facilitate the introduction of innovative treatments, enhancing patient outcomes and driving market growth. The focus on patient-centric care will further shape the landscape, ensuring that treatments are tailored to individual needs.
| Segment | Sub-Segments |
|---|---|
| By Therapy Type | Chemotherapy Immunotherapy Radiation Therapy Surgery Targeted Therapy Stem Cell Transplant Others (e.g., Combination Therapies, Supportive Care) |
| By Treatment Stage | Early Stage Intermediate Stage Advanced/Relapsed Stage |
| By End-User | Hospitals Specialty Cancer Centers Research & Academic Institutes |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies & Drug Stores Online Pharmacies |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Patient Demographics | Age Group (Infants, Toddlers, Children) Gender |
| By Treatment Duration | Short-term Treatment Long-term Treatment Maintenance Therapy |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pediatric Oncologists | 60 | Oncology Specialists, Treatment Protocol Directors |
| Hospital Administrators | 50 | Healthcare Executives, Financial Officers |
| Clinical Researchers | 40 | Research Scientists, Clinical Trial Coordinators |
| Caregivers of Neuroblastoma Patients | 45 | Parents, Guardians, Patient Advocates |
| Pharmaceutical Representatives | 40 | Sales Managers, Product Specialists |
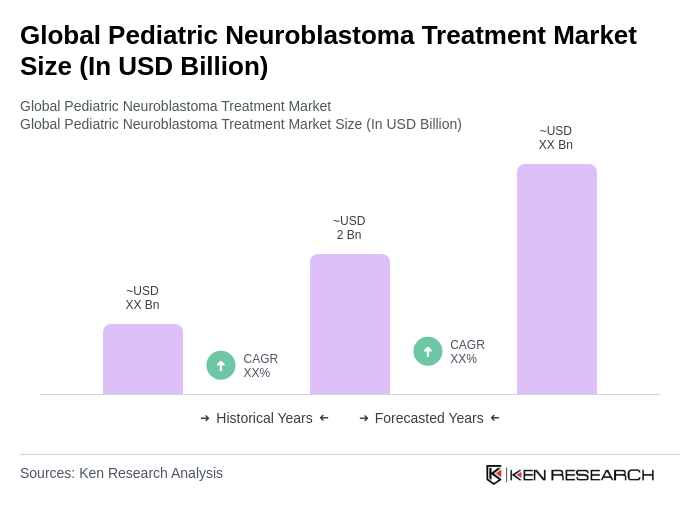
The Global Pediatric Neuroblastoma Treatment Market is valued at approximately USD 2 billion, reflecting a significant growth driven by increasing incidence rates, advancements in therapy methods, and enhanced access to treatment through global health initiatives.