Region:Global
Author(s):Shubham
Product Code:KRAA1708
Pages:87
Published On:August 2025
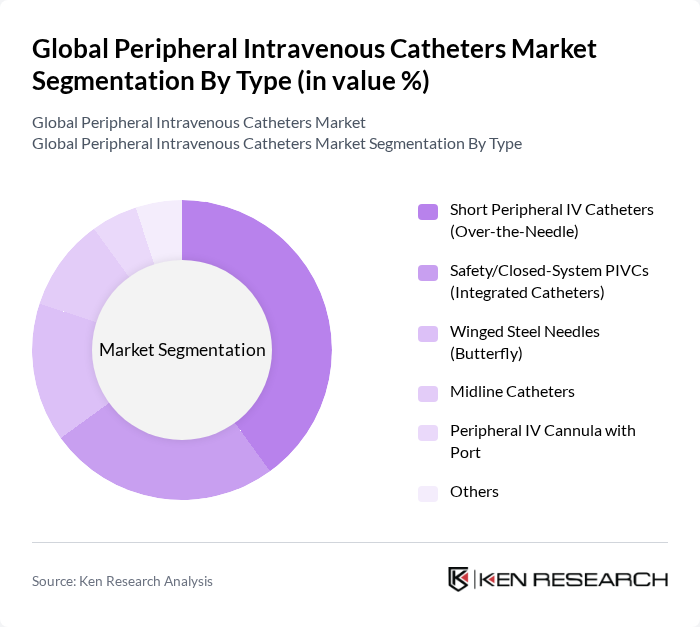
By Type:The market is segmented into various types of peripheral intravenous catheters, including Short Peripheral IV Catheters (Over-the-Needle), Safety/Closed-System PIVCs (Integrated Catheters), Winged Steel Needles (Butterfly), Midline Catheters, Peripheral IV Cannula with Port, and Others. Among these, Short Peripheral IV Catheters are the most widely used due to their ease of use and effectiveness in short-term intravenous therapy, with the peripheral segment being the largest within the overall IV catheter market. The demand for Safety/Closed-System PIVCs is increasing as providers prioritize needlestick injury prevention, stabilization, and infection control, with integrated designs associated with workflow efficiency and lower contamination risk .
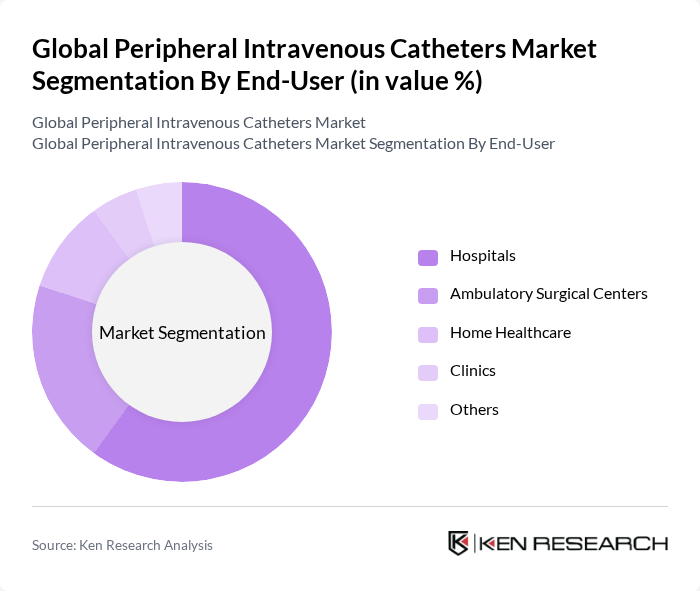
By End-User:The end-user segmentation includes Hospitals, Ambulatory Surgical Centers, Home Healthcare, Clinics, and Others. Hospitals are the primary end-users of peripheral intravenous catheters due to high inpatient volumes and frequent need for IV therapy. Ambulatory Surgical Centers continue to grow with the shift to outpatient procedures, and Home Healthcare is expanding as payors and providers favor care-at-home models, which supports more peripheral IV use outside hospitals .
The Global Peripheral Intravenous Catheters Market is characterized by a dynamic mix of regional and international players. Leading participants such as B. Braun SE, Smiths Medical (ICU Medical, Inc.), Becton, Dickinson and Company (BD), Teleflex Incorporated, Terumo Corporation, Cardinal Health, Inc., Fresenius Kabi AG, Vygon SA, Cook Medical LLC, Nipro Corporation, AngioDynamics, Inc., Baxter International Inc., 3M Company, Poly Medicure Ltd. (Polymed), B. Braun’s StarSystem and Introcan Safety (Product Families) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the peripheral intravenous catheters market appears promising, driven by ongoing advancements in technology and an increasing focus on patient-centered care. As healthcare systems evolve, the integration of smart technologies and telemedicine is expected to enhance catheter management and monitoring. Furthermore, the growing emphasis on home healthcare solutions will likely lead to increased demand for user-friendly catheters, enabling patients to receive treatment in the comfort of their homes while minimizing hospital visits and associated costs.(General directional statements retained; no quantitative projections added.)
| Segment | Sub-Segments |
|---|---|
| By Type | Short Peripheral IV Catheters (Over-the-Needle) Safety/Closed-System PIVCs (Integrated Catheters) Winged Steel Needles (Butterfly) Midline Catheters Peripheral IV Cannula with Port Others |
| By End-User | Hospitals Ambulatory Surgical Centers Home Healthcare Clinics Others |
| By Application | Oncology Emergency and Critical Care Fluid and Medication Administration Antibiotic and Antiviral Therapy Others |
| By Material | Polyurethane Fluorinated Ethylene Propylene (FEP)/Teflon Silicone Others |
| By Length | Short Catheters Midline-Length Catheters Others |
| By Distribution Channel | Direct Sales to Providers Medical Distributors Group Purchasing Organizations (GPOs) Online Sales Others |
| By Price Range | Economy Standard Premium (Safety/Integrated & Antimicrobial) Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Procurement Departments | 120 | Procurement Managers, Supply Chain Directors |
| Clinical Staff in Emergency Departments | 100 | Nurses, Emergency Medicine Physicians |
| IV Catheter Manufacturers | 80 | Product Managers, R&D Engineers |
| Healthcare Policy Makers | 60 | Health Economists, Regulatory Affairs Specialists |
| Patient Advocacy Groups | 40 | Patient Representatives, Healthcare Advocates |
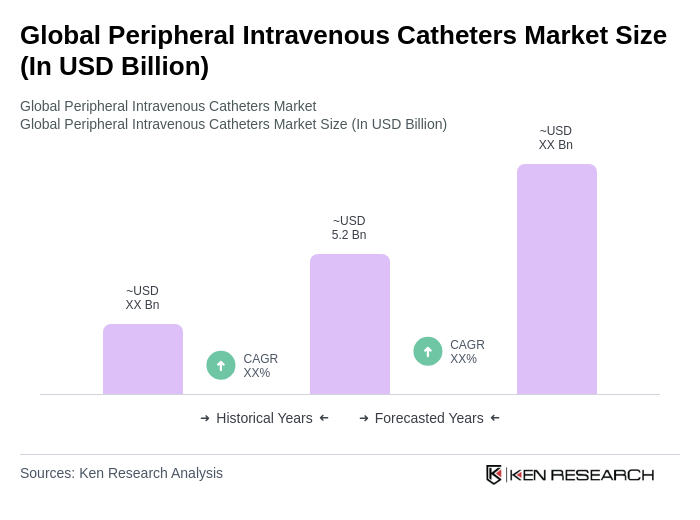
The Global Peripheral Intravenous Catheters Market is valued at approximately USD 5.2 billion, with estimates suggesting it could reach around USD 5.4 billion. This growth is driven by increasing demand from hospitals and outpatient care settings.