Region:Global
Author(s):Shubham
Product Code:KRAC0741
Pages:91
Published On:August 2025
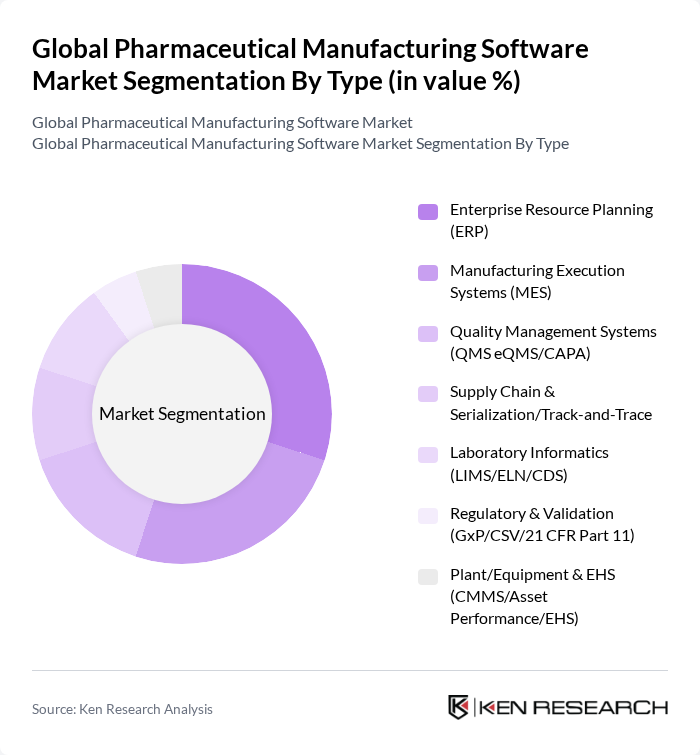
By Type:The market is segmented into various types of software solutions that cater to different aspects of pharmaceutical manufacturing. The primary subsegments include Enterprise Resource Planning (ERP), Manufacturing Execution Systems (MES), Quality Management Systems (QMS eQMS/CAPA), Supply Chain & Serialization/Track-and-Trace, Laboratory Informatics (LIMS/ELN/CDS), Regulatory & Validation (GxP/CSV/21 CFR Part 11), and Plant/Equipment & EHS (CMMS/Asset Performance/EHS). Industry sources consistently list MES, ERP, QMS, LIMS, and regulatory compliance as core categories in this market. ERP systems remain widely adopted for integrating business and manufacturing processes, while MES and eQMS are seeing strong adoption as pharma manufacturers digitize shop-floor execution, electronic batch records, deviations/CAPA, and release processes to meet GMP and data integrity requirements .
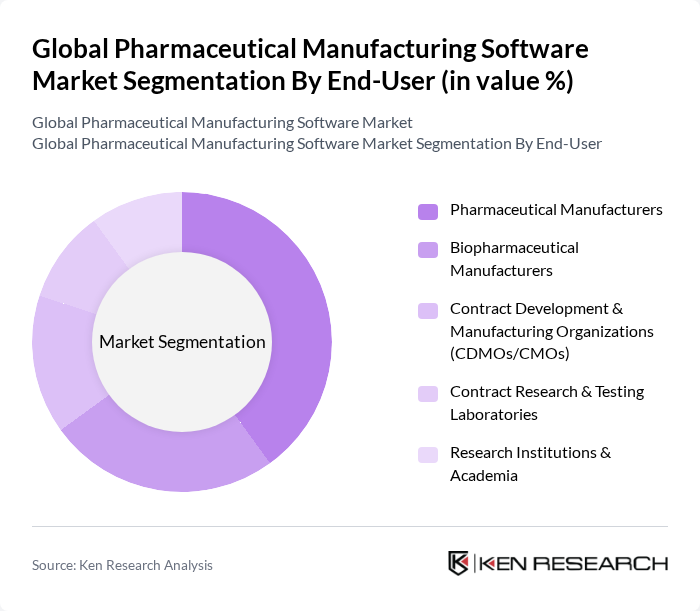
By End-User:The end-user segmentation includes Pharmaceutical Manufacturers, Biopharmaceutical Manufacturers, Contract Development & Manufacturing Organizations (CDMOs/CMOs), Contract Research & Testing Laboratories, and Research Institutions & Academia. Pharmaceutical Manufacturers dominate this segment given their large installed bases of regulated facilities and sustained investments in MES, ERP, QMS, and LIMS to manage complex, validated processes and ensure release compliance; growing biopharma capacity expansions further support demand across MES/eBR, PAT, and analytics .
The Global Pharmaceutical Manufacturing Software Market is characterized by a dynamic mix of regional and international players. Leading participants such as SAP SE, Oracle Corporation, Siemens AG (Siemens Digital Industries Software), Dassault Systèmes SE, Veeva Systems Inc., MasterControl Inc., PTC Inc., Medidata Solutions, a Dassault Systèmes company, QAD Inc. (QAD Adaptive ERP), LabWare, Inc., Werum IT Solutions (PAS-X, Körber Business Area Pharma), Honeywell International Inc. (Honeywell Forge/Experion for Life Sciences), Rockwell Automation, Inc. (FactoryTalk PharmaSuite), Aspen Technology, Inc. (AspenTech), Aizon contribute to innovation, geographic expansion, and service delivery in this space, with cloud deployment, analytics/AI, data integrity, and connected shop-floor integrations frequently cited as key adoption themes in recent research .
Enhancements to growth drivers and trends (validated):
The future of pharmaceutical manufacturing software is poised for transformative growth, driven by technological advancements and evolving industry needs. As companies increasingly adopt artificial intelligence and machine learning, operational efficiencies are expected to improve significantly. Furthermore, the shift towards personalized medicine will necessitate more sophisticated software solutions, enabling tailored production processes. The emphasis on sustainability will also shape software development, as manufacturers seek eco-friendly practices and compliance with environmental regulations, ensuring a robust and adaptive market landscape.
| Segment | Sub-Segments |
|---|---|
| By Type | Enterprise Resource Planning (ERP) Manufacturing Execution Systems (MES) Quality Management Systems (QMS eQMS/CAPA) Supply Chain & Serialization/Track-and-Trace Laboratory Informatics (LIMS/ELN/CDS) Regulatory & Validation (GxP/CSV/21 CFR Part 11) Plant/Equipment & EHS (CMMS/Asset Performance/EHS) |
| By End-User | Pharmaceutical Manufacturers Biopharmaceutical Manufacturers Contract Development & Manufacturing Organizations (CDMOs/CMOs) Contract Research & Testing Laboratories Research Institutions & Academia |
| By Deployment Mode | On-Premises Cloud (SaaS) Hybrid |
| By Functionality | Production Planning & Scheduling Inventory, Warehouse & Cold Chain Compliance, Validation & Audit Trails Electronic Batch Records (EBR) & Batch Release Analytics, AI/ML & Real-Time Monitoring |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Company Size | Large Enterprises Medium Enterprises Small Enterprises |
| By Pricing Model | Subscription (Per-User/Per-Site) Perpetual License + AMC Usage-Based (Consumption/Device) Enterprise Agreements |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Pharmaceutical Manufacturing Software Users | 120 | IT Managers, Production Supervisors |
| Quality Assurance Software Implementers | 90 | QA Managers, Compliance Officers |
| ERP System Users in Pharma | 70 | Operations Managers, Financial Analysts |
| Regulatory Compliance Software Users | 60 | Regulatory Affairs Specialists, Legal Advisors |
| Supply Chain Management Software Users | 80 | Supply Chain Directors, Logistics Coordinators |
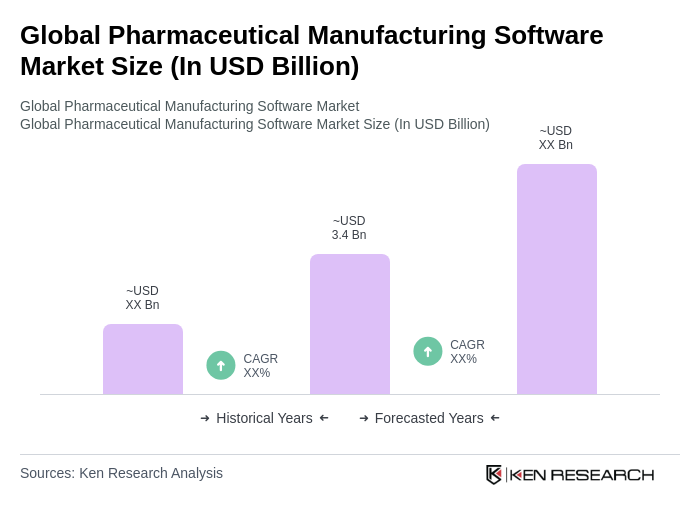
The Global Pharmaceutical Manufacturing Software Market is valued at approximately USD 3.4 billion, reflecting a significant increase in the adoption of various software solutions such as MES, ERP, QMS, and LIMS within the pharmaceutical manufacturing sector.