Region:Global
Author(s):Dev
Product Code:KRAD0396
Pages:95
Published On:August 2025
By Type:The market is segmented into various types of retinal implants, including epiretinal implants, subretinal implants, suprachoroidal implants, and implantable miniature telescopes. Epiretinal implants such as the Argus II established the early clinical paradigm, but their commercial footprint has contracted; subretinal implants (e.g., Alpha AMS/PRIMA concepts) have gained momentum in trials, particularly for atrophic age-related macular degeneration, where photovoltaic subretinal approaches are being clinically evaluated. IMT devices continue to serve late?stage AMD through magnification rather than neural stimulation, remaining part of the broader vision prosthesis landscape .

By End-User:The end-user segmentation includes hospitals, specialty eye centers, academic and research institutions, and manufacturers’ clinical partners. Hospitals dominate due to comprehensive surgical capabilities and established perioperative care. Specialty eye centers contribute significantly as hubs for complex retinal procedures and implant evaluations. Academic and research institutions play a key role in early-stage clinical studies and technology translation .

The Global Retinal Implants Market is characterized by a dynamic mix of regional and international players. Leading participants such as Second Sight Medical Products, Inc. (legacy Argus II), Retina Implant AG, Pixium Vision S.A., Bionic Vision Technologies Pty Ltd, VisionCare Ophthalmic Technologies, Inc. (Implantable Miniature Telescope), iBionics, Vivani Medical, Inc. (formerly Second Sight interface assets), Nidek Co., Ltd., Carl Zeiss Meditec AG, Alcon Inc., Johnson & Johnson Vision, Novartis AG, Santen Pharmaceutical Co., Ltd., Heidelberg Engineering GmbH, Optobionics Corporation contribute to innovation, geographic expansion, and service delivery in this space.
The future of the retinal implants market appears promising, driven by ongoing technological advancements and an increasing focus on patient-centered care. As healthcare systems adapt to the growing geriatric population, the integration of minimally invasive techniques and artificial intelligence in surgical procedures is expected to enhance treatment efficacy. Furthermore, the expansion of telemedicine in eye care will facilitate access to specialized services, ensuring that more patients receive timely interventions and improving overall health outcomes in the sector.
| Segment | Sub-Segments |
|---|---|
| By Type | Epiretinal Implants (e.g., Argus II) Subretinal Implants (e.g., Alpha AMS/Alpha AMS PRO) Suprachoroidal Implants Implantable Miniature Telescope (IMT) and Other Vision Prostheses |
| By End-User | Hospitals Specialty Eye Centers/Outpatient Facilities Academic & Research Institutions Manufacturers’ Clinical Partners |
| By Application | Retinitis Pigmentosa Age-related Macular Degeneration (AMD) Inherited Retinal Dystrophies (non-RP) Clinical Research/Trials |
| By Distribution Channel | Direct Sales to Providers Authorized Distributors Tender/Institutional Procurement Cross-border/Export Programs |
| By Region | North America Europe Asia-Pacific Latin America |
| By Price Range | Premium Mid-range Economy |
| By Patient Demographics | Pediatric Adult Geriatric Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Ophthalmology Clinics | 110 | Ophthalmologists, Clinic Managers |
| Hospital Surgical Departments | 90 | Surgeons, Medical Directors |
| Medical Device Manufacturers | 75 | Product Managers, R&D Heads |
| Healthcare Policy Makers | 50 | Health Economists, Policy Analysts |
| Patient Advocacy Groups | 60 | Advocacy Leaders, Patient Representatives |
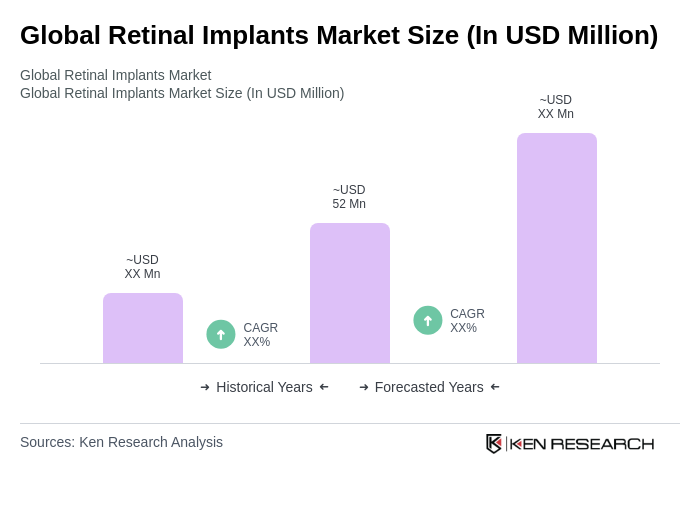
The Global Retinal Implants Market is valued at approximately USD 52 million, reflecting its niche and procedure-driven nature, with limited commercial availability across various regions.