Region:Global
Author(s):Shubham
Product Code:KRAA1820
Pages:99
Published On:August 2025
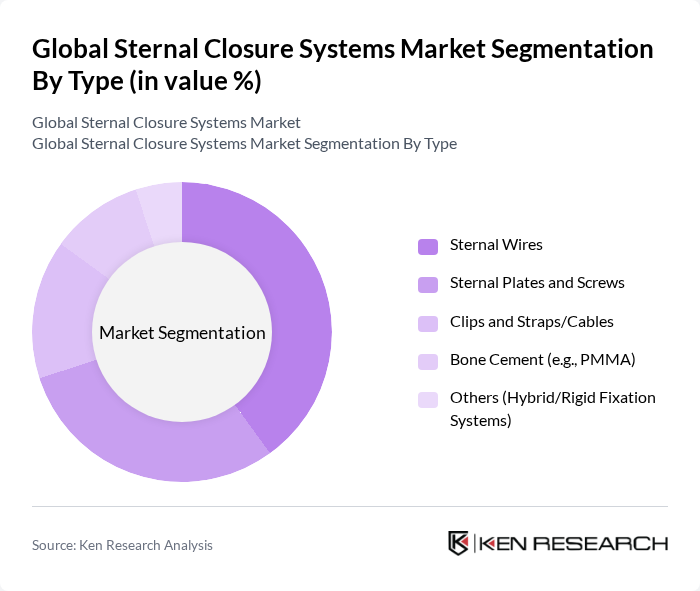
By Type:The market is segmented into various types, including Sternal Wires, Sternal Plates and Screws, Clips and Straps/Cables, Bone Cement (e.g., PMMA), and Others (Hybrid/Rigid Fixation Systems). Among these, Sternal Wires are the most widely used in routine sternotomy closure due to cost-effectiveness and familiarity in cardiac surgery workflows, while rigid fixation with Sternal Plates and Screws is gaining traction for high-risk patients and when greater stability is desired. Demand for Clips and Straps/Cables is supported by their utility as adjuncts or alternatives in specific clinical scenarios, aiming to simplify application and distribute forces across the sternum.
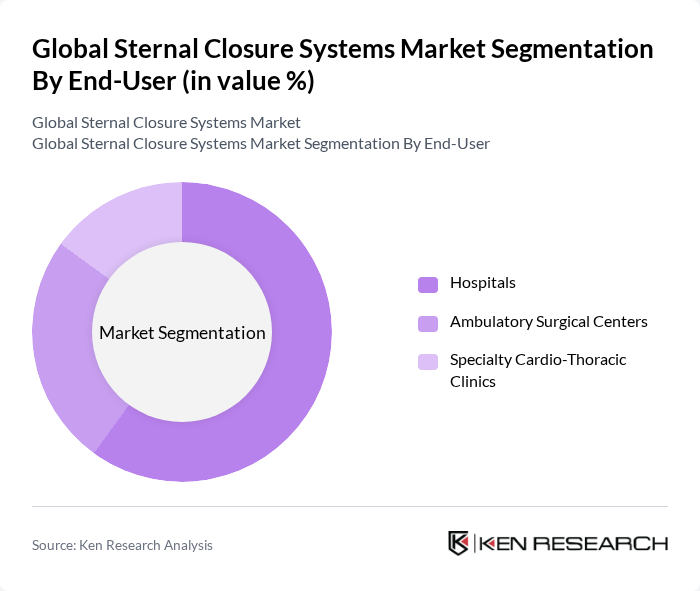
By End-User:The end-user segmentation includes Hospitals, Ambulatory Surgical Centers, and Specialty Cardio-Thoracic Clinics. Hospitals dominate due to concentration of cardiothoracic operating rooms, intensive care resources, and multi-disciplinary teams handling complex sternotomy cases. Ambulatory Surgical Centers are gaining attention for select thoracic procedures and enhanced efficiency where appropriate, while Specialty Cardio-Thoracic Clinics offer focused expertise and integrated perioperative pathways for sternotomy patients.
The Global Sternal Closure Systems Market is characterized by a dynamic mix of regional and international players. Leading participants such as Zimmer Biomet, DePuy Synthes (Johnson & Johnson MedTech), Aesculap (B. Braun Melsungen AG), KLS Martin Group, SternaLock (Zimmer Biomet), Orthofix Medical Inc., Able Medical Devices, Kinamed Inc., Innovative Titanium Solutions (ITS) – Sternal Talon, IDEAR S.R.L., Jeil Medical Corporation, Praesidia S.r.l., Ossirius, MedXpert GmbH, Acumed LLC contribute to innovation, geographic expansion, and service delivery in this space.
The future of the sternal closure systems market in future appears promising, driven by technological advancements and an increasing focus on patient-centric care. As healthcare providers prioritize minimally invasive techniques, the demand for innovative sternal closure solutions is expected to rise. Additionally, the growing investment in healthcare infrastructure will facilitate the adoption of advanced surgical technologies, further enhancing market dynamics and improving patient outcomes in the region.
| Segment | Sub-Segments |
|---|---|
| By Type | Sternal Wires Sternal Plates and Screws Clips and Straps/Cables Bone Cement (e.g., PMMA) Others (Hybrid/Rigid Fixation Systems) |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Cardio-Thoracic Clinics |
| By Material | Titanium Stainless Steel Polyether Ether Ketone (PEEK) and Other Polymers |
| By Procedure | Median Sternotomy Hemisternotomy Bilateral Thoracosternotomy Other Procedures |
| By Distribution Channel | Direct Sales Distributors Group Purchasing Organizations (GPOs) and Tenders |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Fixation Technique | Rigid Fixation (Plates/Screws) Wire/Cable Cerclage Hybrid Fixation |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiac Surgeons | 100 | Cardiothoracic Surgeons, Surgical Directors |
| Hospital Procurement Managers | 80 | Procurement Officers, Supply Chain Managers |
| Operating Room Staff | 70 | Nurses, Surgical Technologists |
| Medical Device Distributors | 60 | Sales Representatives, Distribution Managers |
| Healthcare Policy Makers | 50 | Health Economists, Policy Analysts |
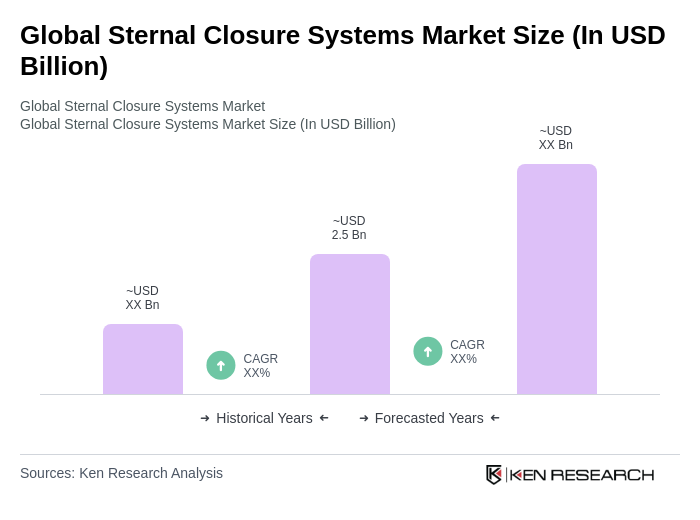
The Global Sternal Closure Systems Market is valued at approximately USD 2.5 billion, reflecting a significant growth driven by the rising prevalence of cardiovascular diseases and advancements in surgical techniques and protocols.