Region:Global
Author(s):Dev
Product Code:KRAD5320
Pages:90
Published On:December 2025
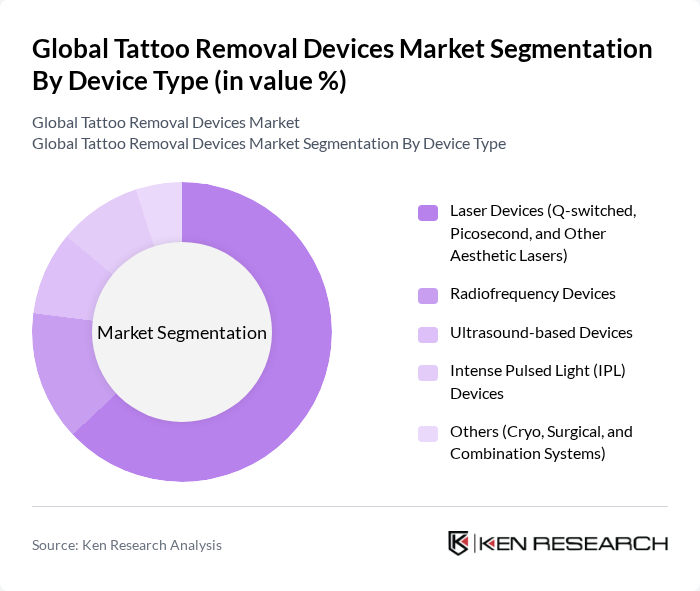
By Device Type:The market is segmented into various device types, including Laser Devices (Q-switched, Picosecond, and Other Aesthetic Lasers), Radiofrequency Devices, Ultrasound-based Devices, Intense Pulsed Light (IPL) Devices, and Others (Cryo, Surgical, and Combination Systems). Laser Devices are the most widely used, with laser platforms representing roughly three?fifths to two?thirds of global tattoo removal device revenues in recent analyses, reflecting their superior effectiveness and precision in targeting tattoo ink chromophores while minimizing injury to surrounding skin. Radiofrequency and ultrasound?based systems remain niche and are often used as adjuncts or in combination protocols rather than stand?alone primary modalities.
By Procedure Type:The procedure types include Laser Tattoo Removal, Radiofrequency-assisted Tattoo Removal, Ultrasound-assisted Tattoo Removal, Surgical Excision and Dermabrasion, and Others (Topical Adjuvants and Combination Protocols). Laser Tattoo Removal is the leading procedure type and is regarded in clinical literature and industry reports as the standard of care for professional and amateur tattoo removal because it is non?invasive, offers selective photothermolysis of ink particles, and is associated with relatively short recovery times compared with surgical excision and dermabrasion. Alternative procedures such as surgical excision and dermabrasion are generally reserved for small tattoos or refractory cases due to higher risks of scarring and longer downtime, while topical and combination protocols are primarily used as adjuncts to laser therapy.

The Global Tattoo Removal Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Cynosure, LLC, Cutera, Inc., Lumenis Ltd., Candela Medical, Alma Lasers Ltd. (Sisram Medical), Sciton, Inc., Quanta System S.p.A., BTL Industries, Lutronic Corporation, LASEROPTEK Co., Ltd., Fotona d.o.o., EL.En. S.p.A. (DEKA M.E.L.A.), Lynton Lasers Ltd., InMode Ltd., Venus Concept Inc. contribute to innovation, geographic expansion, and service delivery in this space, offering portfolios that increasingly emphasize multi?wavelength, picosecond, and fractional technologies aimed at improving clearance of multicolored and resistant tattoos.
The future of the tattoo removal market appears promising, driven by technological advancements and a growing consumer base seeking effective solutions. As awareness of skin health continues to rise, more individuals are likely to pursue professional removal services. Additionally, the integration of non-invasive techniques and eco-friendly products is expected to attract environmentally conscious consumers, further expanding the market. The focus on patient education will also enhance consumer confidence, leading to increased demand for tattoo removal procedures.
| Segment | Sub-Segments |
|---|---|
| By Device Type | Laser Devices (Q-switched, Picosecond, and Other Aesthetic Lasers) Radiofrequency Devices Ultrasound-based Devices Intense Pulsed Light (IPL) Devices Others (Cryo, Surgical, and Combination Systems) |
| By Procedure Type | Laser Tattoo Removal Radiofrequency-assisted Tattoo Removal Ultrasound-assisted Tattoo Removal Surgical Excision and Dermabrasion Others (Topical Adjuvants and Combination Protocols) |
| By End-Use | Dermatology Clinics Medical Spas & Beauty Centers Hospitals Specialty Aesthetic Centers & Chains Others (Academic & Research Settings) |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Age Group of Patients | –24 Years –34 Years –44 Years Years and Above Others (Below 18, With Guardian Consent) |
| By Tattoo Characteristics | Professional Tattoos Amateur Tattoos Cosmetic and Medical Tattoos Multicolored Tattoos Traumatic Tattoos |
| By Treatment Regimen | Single-session (High-energy) Treatments Multi-session (Fractionated / Staged) Treatments Combination & Adjunctive Protocols |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Dermatology Clinics | 90 | Dermatologists, Clinic Managers |
| Tattoo Removal Centers | 75 | Clinic Owners, Treatment Specialists |
| Consumer Insights | 140 | Individuals who have undergone tattoo removal |
| Medical Device Manufacturers | 65 | Product Development Managers, Sales Executives |
| Regulatory Bodies | 45 | Health Policy Analysts, Compliance Officers |
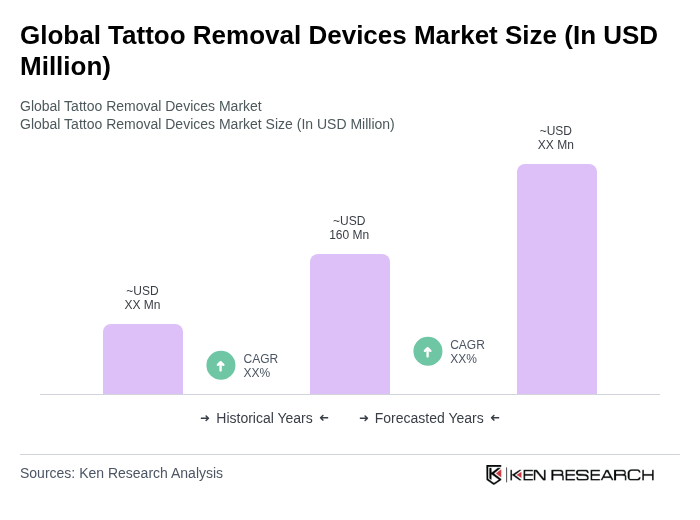
The Global Tattoo Removal Devices Market is valued at approximately USD 160 million, reflecting a significant demand for tattoo removal services driven by factors such as increasing tattoo regret and advancements in laser technologies.