Region:Global
Author(s):Shubham
Product Code:KRAC0609
Pages:83
Published On:August 2025
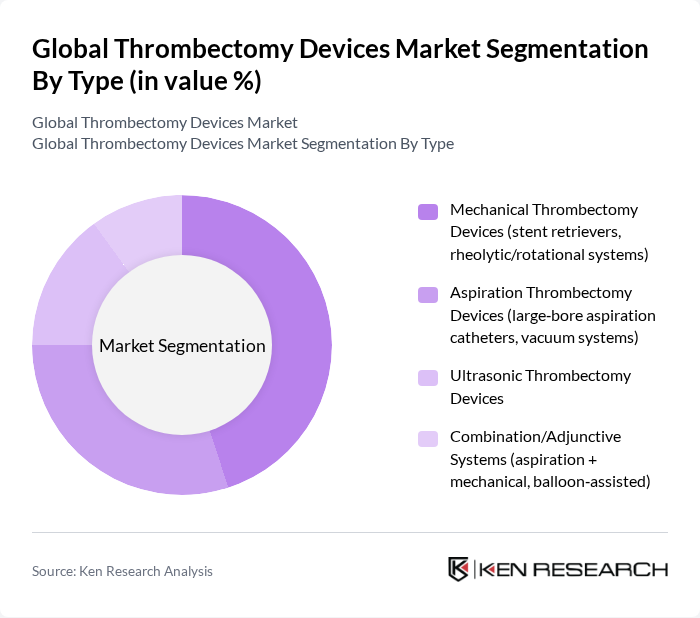
By Type:The thrombectomy devices market is segmented into various types, including Mechanical Thrombectomy Devices, Aspiration Thrombectomy Devices, Ultrasonic Thrombectomy Devices, and Combination/Adjunctive Systems. Among these, Mechanical Thrombectomy Devices, which include stent retrievers and rheolytic systems, are currently leading the market due to their effectiveness in treating ischemic strokes and established clinical outcomes for large vessel occlusion . The increasing adoption of these devices in hospitals and specialized clinics is driven by their proven clinical outcomes and the growing number of stroke cases globally .
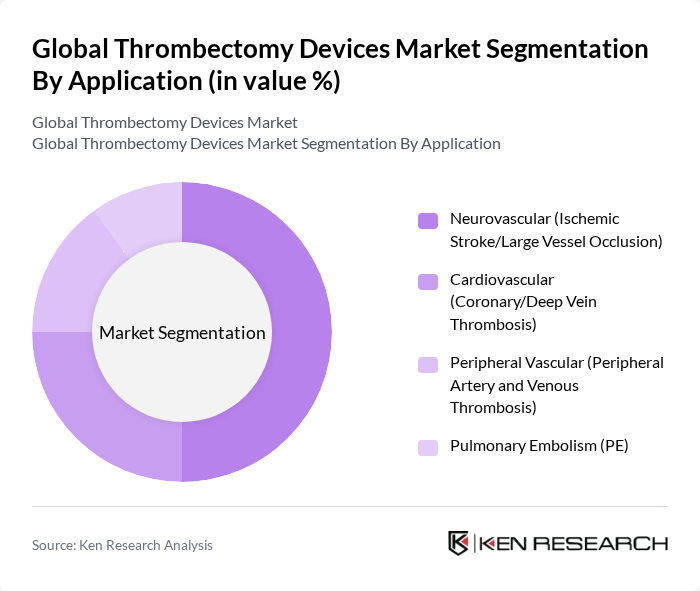
By Application:The market is also segmented by application, which includes Neurovascular, Cardiovascular, Peripheral Vascular, and Pulmonary Embolism. The Neurovascular segment, particularly for ischemic stroke and large vessel occlusion, is the most significant due to the rising incidence of strokes and the effectiveness of thrombectomy procedures in improving patient outcomes . The increasing awareness of stroke management and the availability of advanced thrombectomy devices are further propelling this segment's growth .
The Global Thrombectomy Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Boston Scientific Corporation, Stryker Corporation, Johnson & Johnson MedTech (Cerenovus), Penumbra, Inc., Terumo Corporation, Abbott Laboratories, B. Braun Melsungen AG, Cook Medical LLC, AngioDynamics, Inc., Merit Medical Systems, Inc., Inari Medical, Inc., Asahi Intecc Co., Ltd., MicroPort Scientific Corporation, Acandis GmbH contribute to innovation, geographic expansion, and service delivery in this space.
The future of the thrombectomy devices market appears promising, driven by ongoing technological advancements and an increasing focus on patient-centric care. As healthcare systems evolve, there is a notable shift towards outpatient procedures, which are expected to enhance patient convenience and reduce hospital stays. Additionally, the integration of artificial intelligence and robotics in thrombectomy procedures is anticipated to improve precision and outcomes, further propelling market growth in future.
| Segment | Sub-Segments |
|---|---|
| By Type | Mechanical Thrombectomy Devices (stent retrievers, rheolytic/rotational systems) Aspiration Thrombectomy Devices (large?bore aspiration catheters, vacuum systems) Ultrasonic Thrombectomy Devices Combination/Adjunctive Systems (aspiration + mechanical, balloon?assisted) |
| By Application | Neurovascular (Ischemic Stroke/Large Vessel Occlusion) Cardiovascular (Coronary/Deep Vein Thrombosis) Peripheral Vascular (Peripheral Artery and Venous Thrombosis) Pulmonary Embolism (PE) |
| By End-User | Hospitals (Comprehensive Stroke Centers, Tertiary Care) Ambulatory Surgical Centers Specialty Clinics (Neuro?interventional, Vascular) Others (Academic/Research Institutes) |
| By Distribution Channel | Direct Sales to Providers Distributors/Group Purchasing Organizations (GPOs) Online/Procurement Portals Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Price Range | Economy (entry models, limited accessories) Mid-Range (mainstream configurations) Premium (advanced catheters, coated/stent retrievers, PE systems) |
| By Product Lifecycle Stage | Introduction Stage Growth Stage Maturity Stage Decline Stage |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Interventional Radiology Practices | 100 | Interventional Radiologists, Clinical Directors |
| Vascular Surgery Departments | 80 | Vascular Surgeons, Surgical Coordinators |
| Hospital Procurement Teams | 70 | Procurement Managers, Supply Chain Analysts |
| Medical Device Distributors | 60 | Sales Representatives, Distribution Managers |
| Clinical Research Organizations | 40 | Clinical Research Coordinators, Data Analysts |
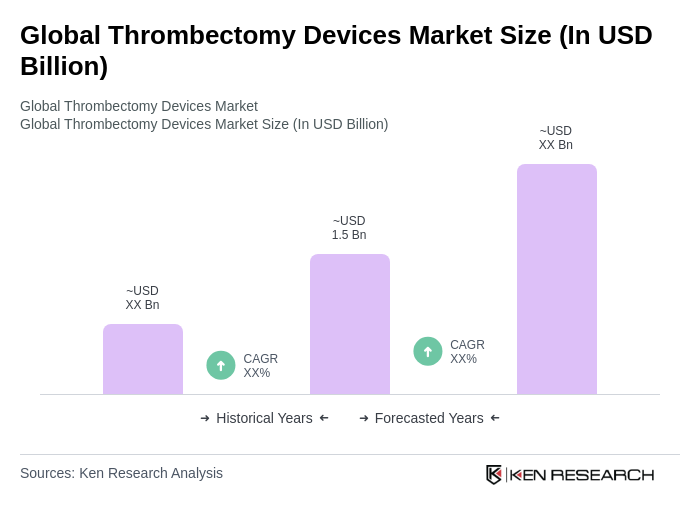
The Global Thrombectomy Devices Market is valued at approximately USD 1.5 billion, driven by the rising prevalence of thromboembolic disorders and advancements in medical technology. This market is expected to grow further as awareness of thrombectomy procedures increases.