Region:Global
Author(s):Shubham
Product Code:KRAA1727
Pages:93
Published On:August 2025
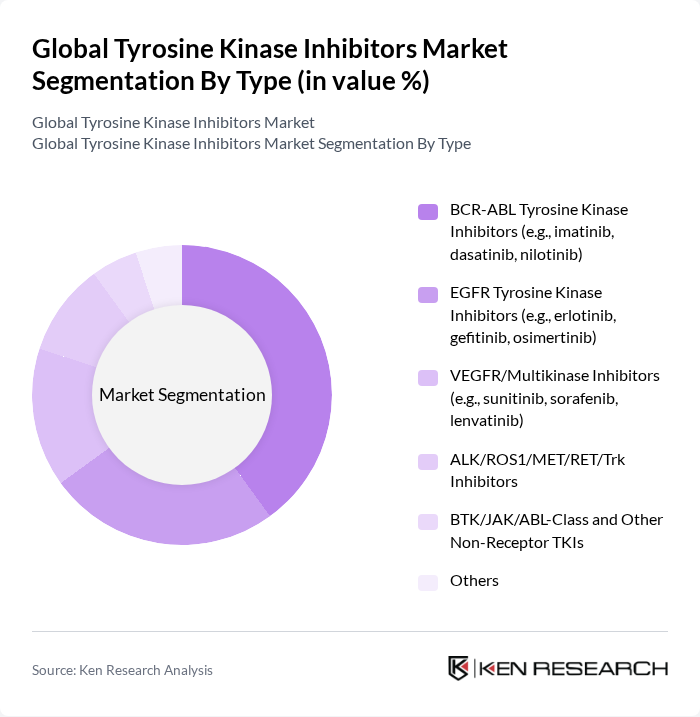
By Type:The market is segmented into various types of tyrosine kinase inhibitors, each targeting specific pathways involved in cancer progression. The leading sub-segment is the BCR-ABL Tyrosine Kinase Inhibitors, which includes drugs like imatinib, dasatinib, and nilotinib. These inhibitors are widely used in treating chronic myeloid leukemia (CML) and have shown significant efficacy, driving their demand in the market.
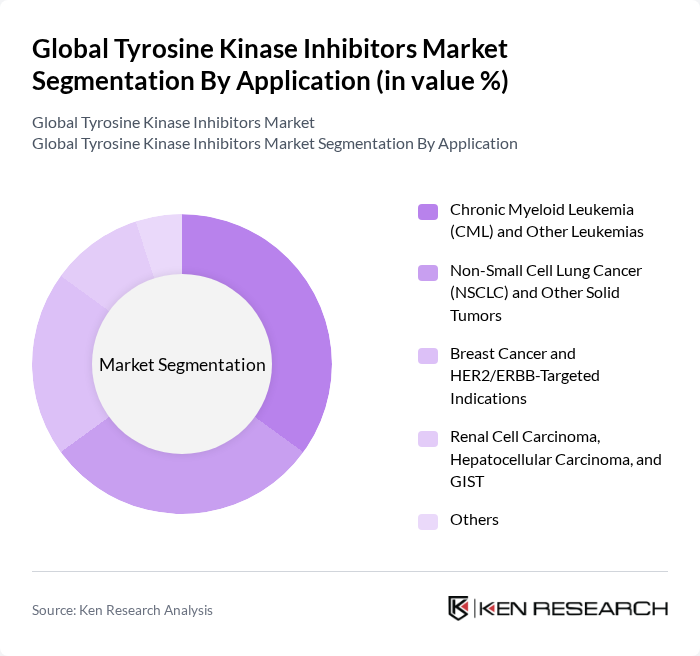
By Application:The application segment of the market includes various cancer types treated with tyrosine kinase inhibitors. The leading application is Chronic Myeloid Leukemia (CML) and Other Leukemias, which accounts for a significant portion of the market due to the high incidence of these diseases and the effectiveness of targeted therapies in managing them. TKIs are also central in NSCLC with EGFR/ALK/ROS1 alterations, HER2-positive breast cancer (TKIs such as lapatinib, neratinib, tucatinib), and multiple solid tumors with RET, MET, NTRK, and VEGFR targets.
The Global Tyrosine Kinase Inhibitors Market is characterized by a dynamic mix of regional and international players. Leading participants such as Novartis AG, Pfizer Inc., Bristol Myers Squibb Company, F. Hoffmann-La Roche Ltd, AstraZeneca PLC, Bayer AG, Merck & Co., Inc. (MSD), Takeda Pharmaceutical Company Limited, Boehringer Ingelheim International GmbH, Eli Lilly and Company, BeiGene, Ltd., Astellas Pharma Inc., Exelixis, Inc., Daiichi Sankyo Company, Limited, Jiangsu Hengrui Pharmaceuticals Co., Ltd. contribute to innovation, geographic expansion, and service delivery in this space.
The future of the tyrosine kinase inhibitors market appears promising, driven by ongoing advancements in precision medicine and the increasing integration of digital health technologies. As healthcare systems prioritize personalized treatment approaches, the demand for targeted therapies is expected to rise. Additionally, the collaboration between pharmaceutical companies and research institutions will likely accelerate the development of innovative TKIs, enhancing treatment options for patients. This dynamic environment will foster growth and improve patient outcomes in the oncology landscape.
| Segment | Sub-Segments |
|---|---|
| By Type | BCR-ABL Tyrosine Kinase Inhibitors (e.g., imatinib, dasatinib, nilotinib) EGFR Tyrosine Kinase Inhibitors (e.g., erlotinib, gefitinib, osimertinib) VEGFR/Multikinase Inhibitors (e.g., sunitinib, sorafenib, lenvatinib) ALK/ROS1/MET/RET/Trk Inhibitors BTK/JAK/ABL-Class and Other Non-Receptor TKIs Others |
| By Application | Chronic Myeloid Leukemia (CML) and Other Leukemias Non-Small Cell Lung Cancer (NSCLC) and Other Solid Tumors Breast Cancer and HER2/ERBB-Targeted Indications Renal Cell Carcinoma, Hepatocellular Carcinoma, and GIST Others |
| By Route of Administration | Oral Intravenous Others |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Online Pharmacies Others |
| By End-User | Hospitals Specialty Oncology Clinics Homecare Settings Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Price Range | Premium Mid-range Economy Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Oncology Clinics | 120 | Oncologists, Nurse Practitioners |
| Pharmaceutical Companies | 90 | Product Managers, R&D Directors |
| Patient Advocacy Groups | 60 | Patient Representatives, Program Coordinators |
| Healthcare Payers | 50 | Health Economists, Policy Analysts |
| Clinical Research Organizations | 40 | Clinical Trial Managers, Regulatory Affairs Specialists |
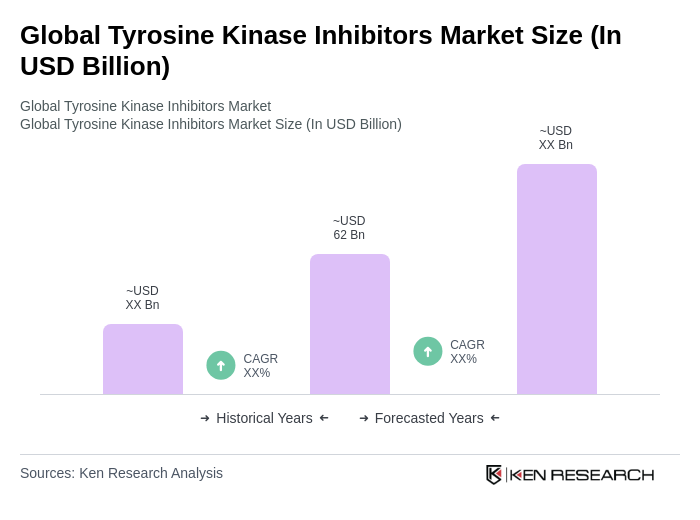
The Global Tyrosine Kinase Inhibitors Market is valued at approximately USD 62 billion, reflecting significant growth driven by the increasing prevalence of cancer and advancements in targeted therapies.