Region:Global
Author(s):Dev
Product Code:KRAA2613
Pages:89
Published On:August 2025
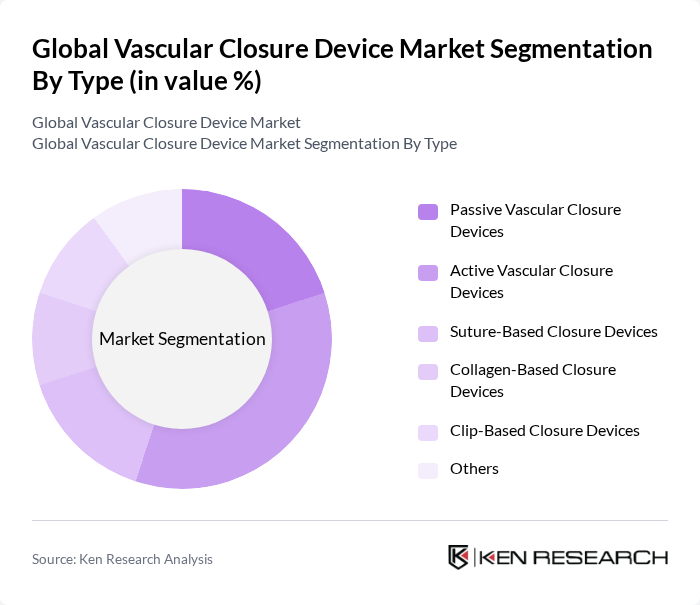
By Type:The vascular closure device market is segmented into Passive Vascular Closure Devices, Active Vascular Closure Devices, Suture-Based Closure Devices, Collagen-Based Closure Devices, Clip-Based Closure Devices, and Others.Active Vascular Closure Devicesare gaining traction due to their effectiveness in reducing time to hemostasis and improving patient outcomes. The demand for these devices is driven by their ability to provide reliable closure with minimal complications, making them a preferred choice in clinical settings. Recent innovations include self-activating mechanisms and integration with imaging guidance, further improving procedural safety and efficiency .
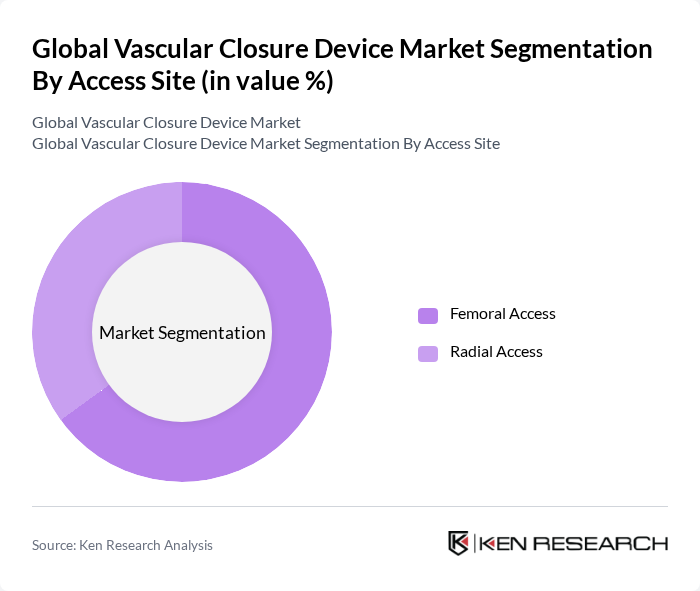
By Access Site:The market is also segmented based on access sites, which include Femoral Access and Radial Access.Femoral Accessremains the dominant approach due to its established efficacy in various procedures, particularly in cardiac interventions. However,Radial Accessis gaining popularity due to its lower complication rates and faster recovery times, appealing to both healthcare providers and patients. The trend toward minimally invasive procedures and outpatient care is further accelerating the adoption of radial access devices .
The Global Vascular Closure Device Market is characterized by a dynamic mix of regional and international players. Leading participants such as Abbott Laboratories, Terumo Corporation, Medtronic plc, Boston Scientific Corporation, Cardinal Health, Inc., W. L. Gore & Associates, Inc., Teleflex Incorporated, Merit Medical Systems, Inc., Avinger, Inc., Vascular Solutions, Inc. (now part of Teleflex), Biomerics, LLC, Cook Medical Incorporated, Koninklijke Philips N.V. (Philips Healthcare), Stryker Corporation, B. Braun Melsungen AG contribute to innovation, geographic expansion, and service delivery in this space.
The future of the vascular closure device market in None is poised for significant transformation, driven by technological advancements and evolving healthcare paradigms. As patient-centric care becomes a priority, the integration of digital health technologies will enhance monitoring and management of vascular procedures. Additionally, the increasing focus on cost-effective healthcare solutions will likely spur innovation in device design and functionality, ensuring that vascular closure devices remain integral to modern surgical practices and outpatient care strategies.
| Segment | Sub-Segments |
|---|---|
| By Type | Passive Vascular Closure Devices Active Vascular Closure Devices Suture-Based Closure Devices Collagen-Based Closure Devices Clip-Based Closure Devices Others |
| By Access Site | Femoral Access Radial Access |
| By End-User | Hospitals Ambulatory Surgical Centers Specialty Clinics Others |
| By Application | Cardiac Procedures Peripheral Procedures Endovascular Procedures Neurovascular Procedures Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Price Range | Low Price Range Mid Price Range High Price Range |
| By Regulatory Approval Status | FDA Approved CE Marked Others |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Cardiovascular Surgery Departments | 100 | Cardiologists, Surgical Team Leaders |
| Hospital Procurement Units | 60 | Procurement Managers, Supply Chain Directors |
| Medical Device Distributors | 50 | Sales Representatives, Distribution Managers |
| Clinical Research Organizations | 40 | Clinical Research Coordinators, Regulatory Affairs Specialists |
| Healthcare Policy Makers | 40 | Health Economists, Policy Analysts |
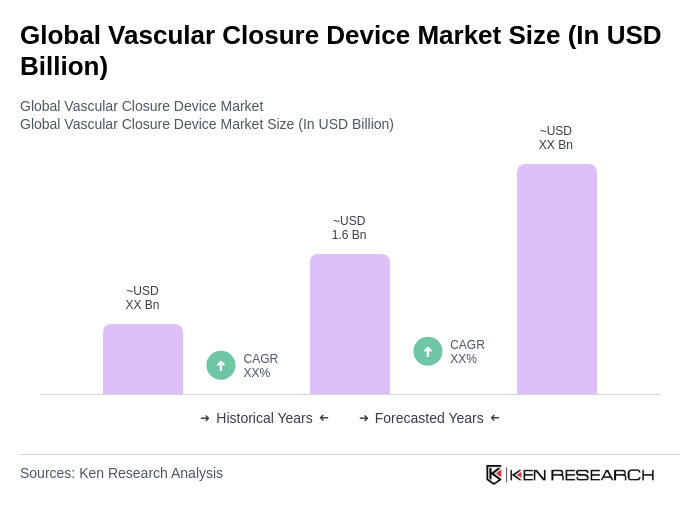
The Global Vascular Closure Device Market is valued at approximately USD 1.6 billion, driven by the increasing prevalence of cardiovascular diseases and advancements in minimally invasive surgical techniques.