Region:Global
Author(s):Shubham
Product Code:KRAD0795
Pages:88
Published On:August 2025
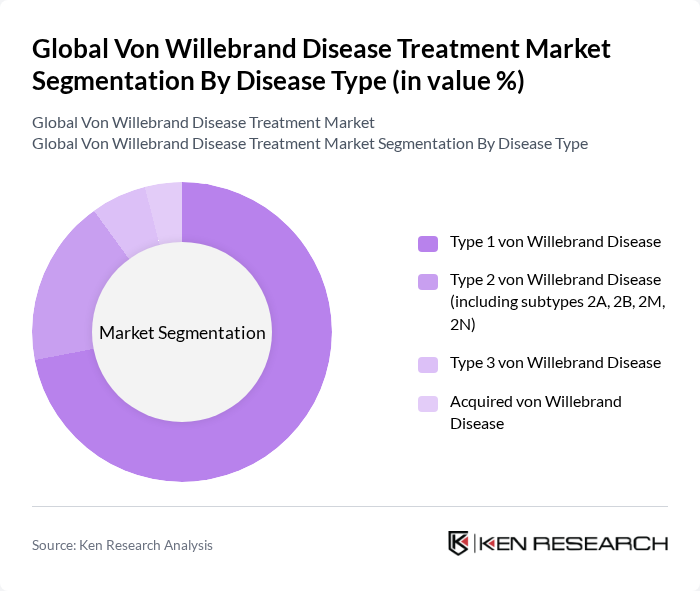
By Disease Type:The market is segmented into four main disease types: Type 1 von Willebrand Disease, Type 2 von Willebrand Disease (including subtypes 2A, 2B, 2M, 2N), Type 3 von Willebrand Disease, and Acquired von Willebrand Disease. Type 1 von Willebrand Disease is the most prevalent, accounting for the majority of diagnosed cases due to its higher detection rates and the availability of effective, non-intensive treatment options.
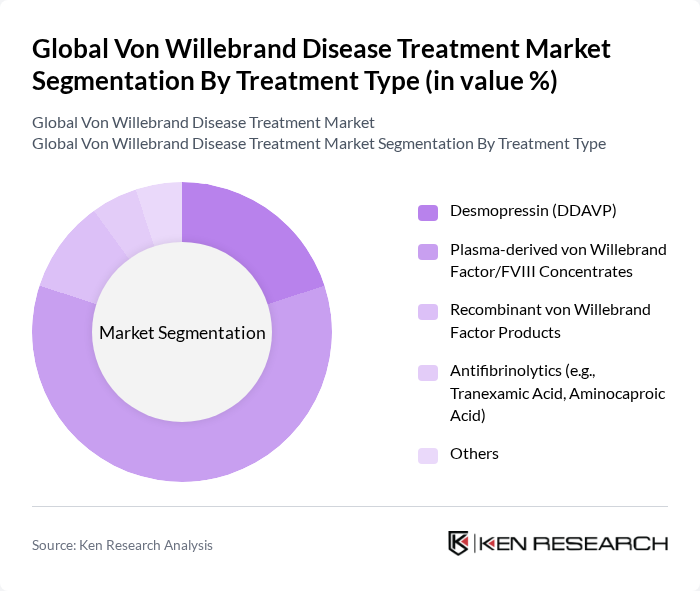
By Treatment Type:The treatment market is categorized into Desmopressin (DDAVP), Plasma-derived von Willebrand Factor/FVIII Concentrates, Recombinant von Willebrand Factor Products, Antifibrinolytics (e.g., Tranexamic Acid, Aminocaproic Acid), and Others. Plasma-derived von Willebrand Factor/FVIII Concentrates remain the primary therapy for moderate to severe cases, while Desmopressin is widely used for mild phenotypes. Recombinant products and antifibrinolytics are gaining traction as adjunct therapies, especially in patients with contraindications to plasma-derived products.
The Global Von Willebrand Disease Treatment Market is characterized by a dynamic mix of regional and international players. Leading participants such as Takeda Pharmaceutical Company Limited, CSL Behring, Octapharma AG, Grifols S.A., Bayer AG, Novo Nordisk A/S, Pfizer Inc., Sanofi S.A., Ferring Pharmaceuticals, BioMarin Pharmaceutical Inc., Sobi AB, LFB S.A., HEMA Biologics, Kedrion S.p.A., Baxter International Inc., American Thrombosis and Hemostasis Network (ATHN), Versiti Blood Center of Wisconsin contribute to innovation, geographic expansion, and service delivery in this space.
The future of the Von Willebrand Disease treatment market is poised for significant transformation, driven by ongoing advancements in personalized medicine and the integration of digital health technologies. As healthcare systems increasingly adopt patient-centric care models, the focus will shift towards tailored treatment plans that enhance patient engagement and adherence. Furthermore, the expansion of telehealth services is expected to improve access to specialized care, particularly in remote areas, thereby fostering a more inclusive healthcare environment for VWD patients.
| Segment | Sub-Segments |
|---|---|
| By Disease Type | Type 1 von Willebrand Disease Type 2 von Willebrand Disease (including subtypes 2A, 2B, 2M, 2N) Type 3 von Willebrand Disease Acquired von Willebrand Disease |
| By Treatment Type | Desmopressin (DDAVP) Plasma-derived von Willebrand Factor/FVIII Concentrates Recombinant von Willebrand Factor Products Antifibrinolytics (e.g., Tranexamic Acid, Aminocaproic Acid) Others |
| By Route of Administration | Intravenous Intranasal Subcutaneous Oral |
| By End-User | Hospitals Specialty Clinics Homecare Settings Others |
| By Distribution Channel | Hospital Pharmacies Retail Pharmacies Online Pharmacies |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Patient Demographics | Pediatric Patients Adult Patients Geriatric Patients |
| By Treatment Setting | Inpatient Outpatient Emergency Care |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hematology Clinics | 100 | Hematologists, Nurse Practitioners |
| Patient Advocacy Groups | 80 | Patient Representatives, Caregivers |
| Pharmaceutical Distributors | 50 | Sales Managers, Product Managers |
| Healthcare Payers | 60 | Policy Analysts, Reimbursement Specialists |
| Clinical Research Organizations | 40 | Clinical Researchers, Regulatory Affairs Specialists |
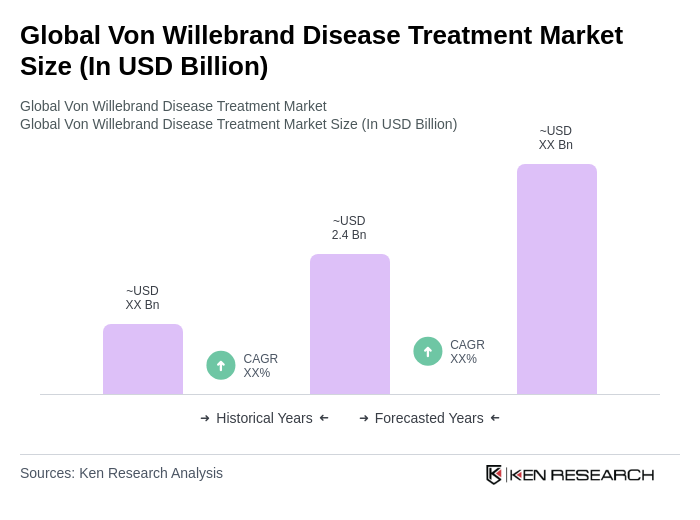
The Global Von Willebrand Disease Treatment Market is valued at approximately USD 2.4 billion, driven by the increasing prevalence of von Willebrand disease and advancements in treatment options and diagnostics.