
APAC Regenerative Medicine Market Outlook to 2030
Region:Asia
Author(s):Mukul
Product Code:KROD3478
October 2024
81
About the Report
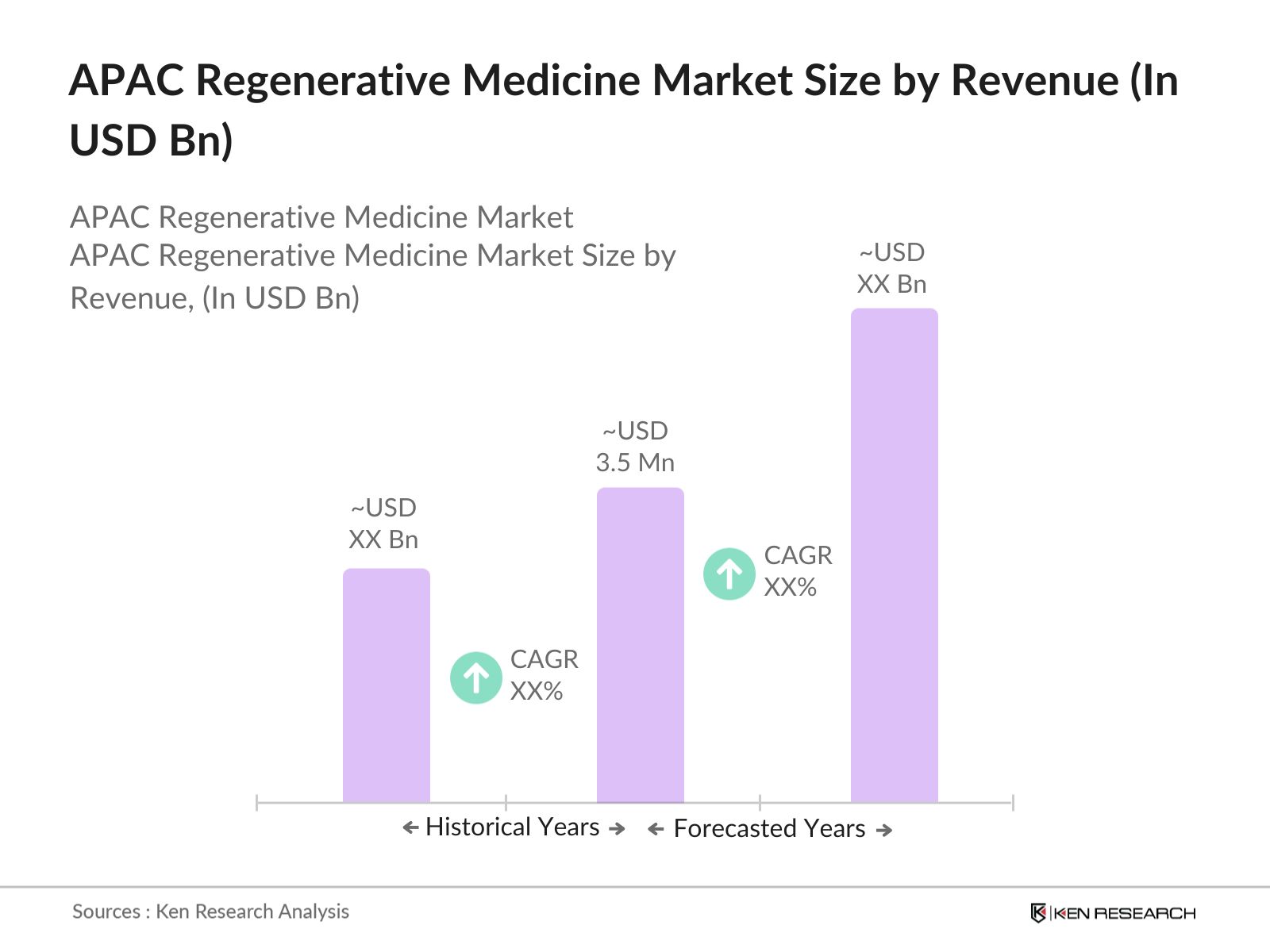
APAC Regenerative Medicine Market Overview
- The APAC regenerative medicine market size by revenue USD 3.5 billion based on a comprehensive five-year historical analysis. This market is driven by a combination of factors, including the increasing prevalence of chronic diseases, a growing elderly population, and rising demand for organ transplantation. Additionally, advancements in stem cell research and gene therapy are key contributors to market growth, along with increased government funding across the region, particularly in countries like Japan and South Korea.
- Japan and China dominate the APAC regenerative medicine market due to their robust healthcare infrastructure, advanced biotechnology research, and favorable government initiatives. Japan is home to some of the worlds leading stem cell research institutes, while China has made significant strides in clinical trials and research, capitalizing on its large population and fast-growing biotech industry. Moreover, regulatory support and investment in life sciences make these countries leaders in the regenerative medicine space.
- The APAC region has seen the introduction of stricter guidelines on stem cell research. In 2023, the Indian Council of Medical Research (ICMR) issued comprehensive guidelines for stem cell research, emphasizing ethical considerations and safety in clinical applications. China has introduced similar regulations, ensuring compliance with international best practices for stem cell use. These guidelines are designed to standardize stem cell research, ensuring that clinical applications meet safety and efficacy standards.
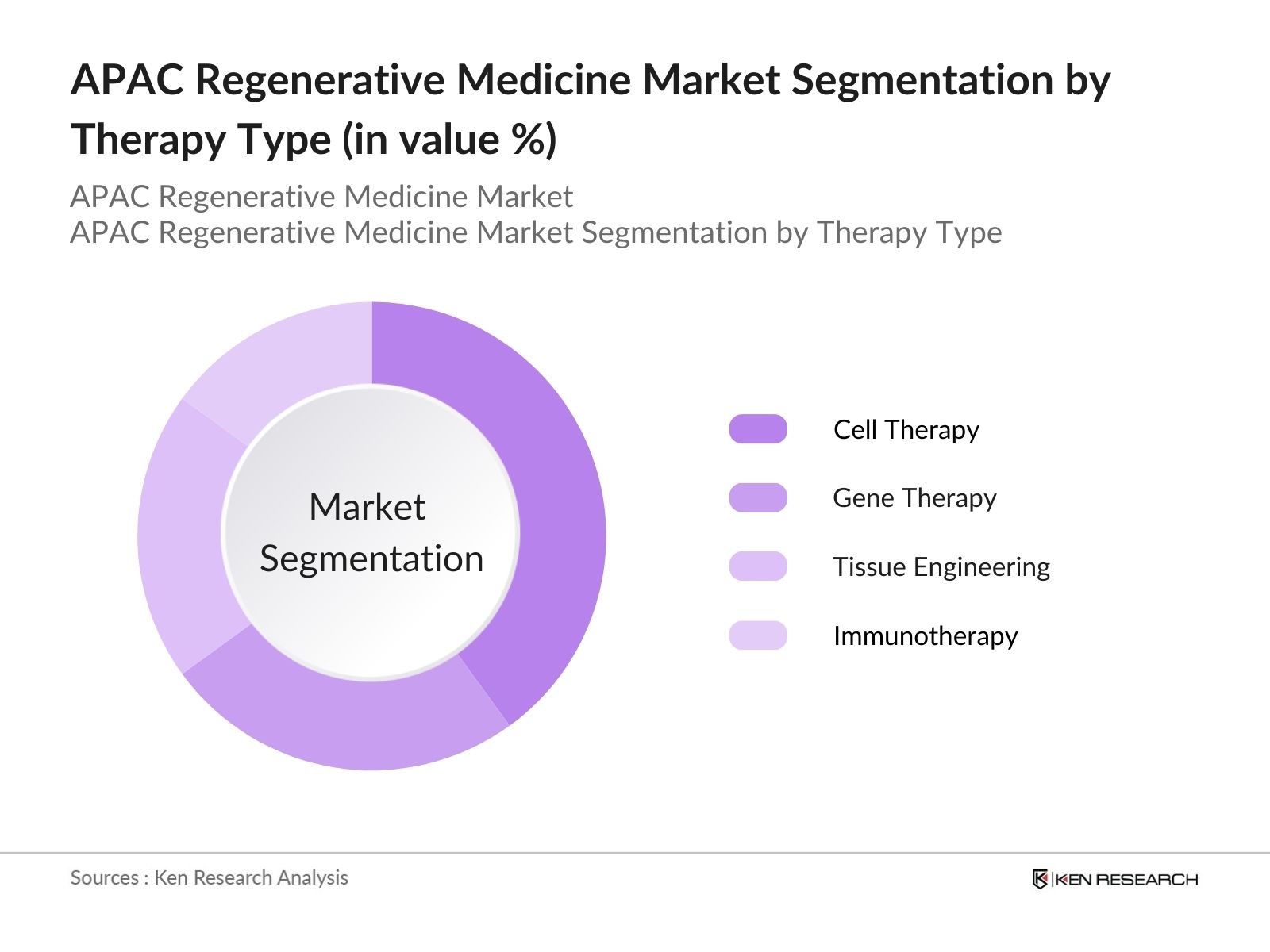
APAC Regenerative Medicine Market Segmentation
- By Therapy Type: The APAC regenerative medicine market is segmented by therapy type into Cell Therapy, Gene Therapy, Tissue Engineering, and Immunotherapy. Cell Therapy holds the dominated the market. The reason for this dominance is its growing application in treating a range of diseases, including cancer and cardiovascular conditions. Companies across the APAC region have invested heavily in R&D to explore the potential of mesenchymal stem cells and induced pluripotent stem cells, further enhancing the adoption of cell-based therapies. Additionally, regulatory approvals in Japan and South Korea for cell therapy products have contributed to its widespread usage.
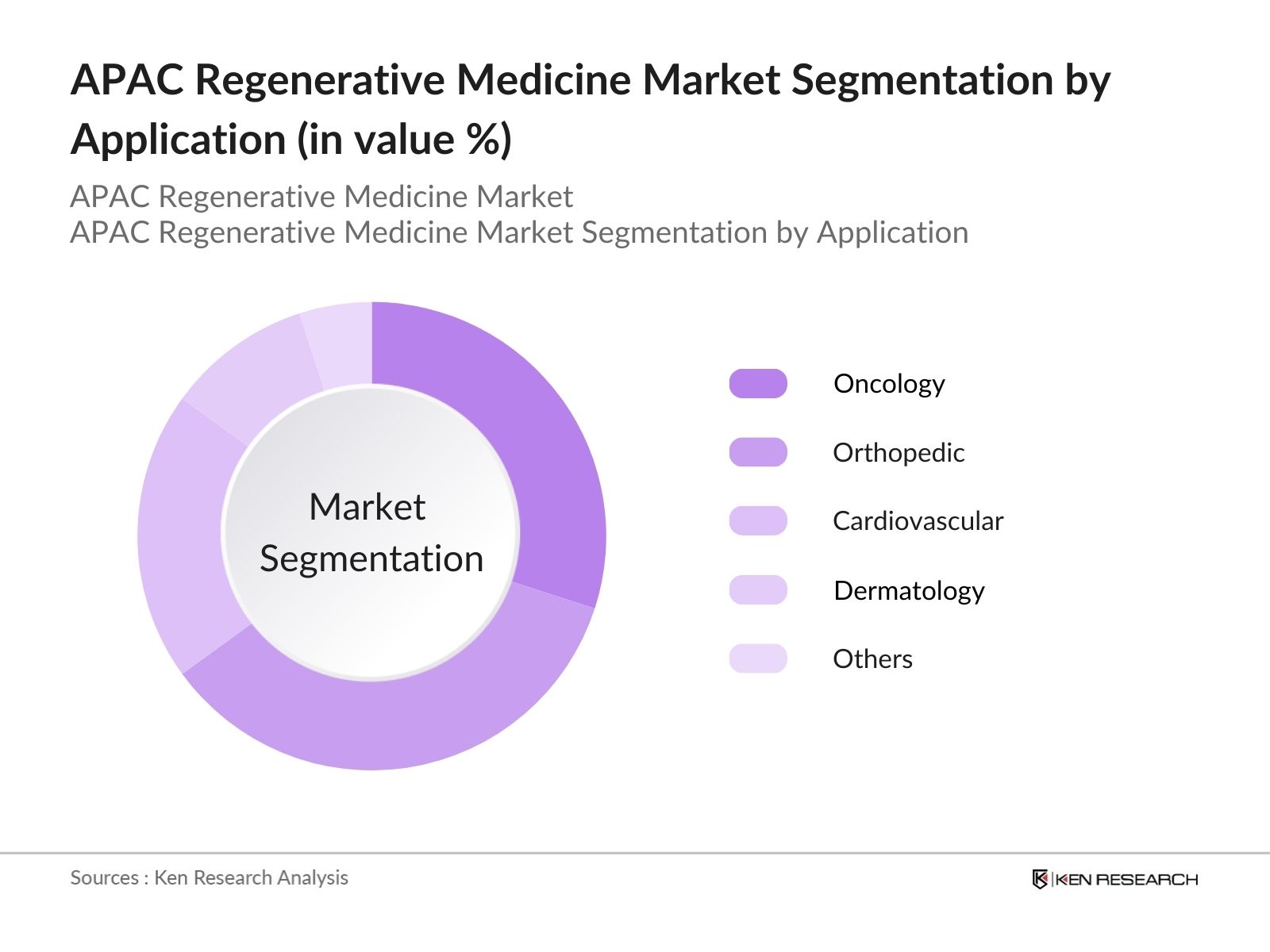
- By Application: The market is also segmented by application into Orthopedic Regenerative Medicine, Oncology Regenerative Medicine, Cardiovascular Regenerative Medicine, Dermatology Regenerative Medicine, and Others (Neurological, Ophthalmology). Oncology Regenerative Medicine is the dominated the market. This is driven by the rising incidence of cancer in the APAC region and the effectiveness of regenerative therapies like CAR-T cell therapies and other gene therapies in combating various forms of cancer. China, Japan, and South Korea have emerged as leaders in oncology-focused regenerative medicine, with multiple ongoing clinical trials and substantial government support for cancer research.
APAC Regenerative Medicine Market Competitive Landscape
The APAC regenerative medicine market is dominated by both regional and global players. These companies focus on technological advancements, strategic collaborations, and investment in R&D to maintain their market leadership. For example, Mesoblast Limited in Australia leads in cell-based therapies, while Japans Fujifilm Holdings Corporation and JCR Pharmaceuticals have a strong presence in gene and cell therapy.
|
Company Name |
Establishment Year |
Headquarters |
Market Focus |
Key Products |
R&D Expenditure |
Strategic Alliances |
Patents Held |
Manufacturing Capacity |
|
JCR Pharmaceuticals Co., Ltd. |
1975 |
Japan |
||||||
|
Fujifilm Holdings Corporation |
1934 |
Japan |
||||||
|
Mesoblast Limited |
2004 |
Australia |
||||||
|
Kolon TissueGene, Inc. |
1999 |
South Korea |
||||||
|
Pharmicell Co., Ltd. |
2002 |
South Korea |
APAC Regenerative Medicine Industry Analysis
APAC Regenerative Medicine Market Growth Drivers
- Rising Prevalence of Chronic Diseases: The APAC region is witnessing a significant rise in the prevalence of chronic diseases, including diabetes, cardiovascular diseases, and cancer. According to the World Health Organization (WHO), Asia accounts for nearly 60% of the global diabetic population, with India and China leading in the number of diabetes cases. This increased incidence is driving the demand for regenerative medicine solutions, particularly in tissue regeneration and stem cell therapies, to address organ damage and loss. The increasing burden of non-communicable diseases is pushing healthcare systems to adopt innovative treatments such as regenerative medicine.
- Increased Government Funding for Regenerative Medicine: Governments across APAC have been allocating substantial funds to accelerate research and development in regenerative medicine. In 2022, the Japanese government announced a 110 billion investment into regenerative medicine, aiming to strengthen its leadership in the global market. Similarly, the Chinese governments 14th Five-Year Plan emphasizes biotech development, allocating 500 billion for the health sector, with a significant portion directed toward regenerative medicine. These investments have catalyzed advancements in clinical trials and commercialization of stem cell therapies in the region.
- Aging Population and Rising Demand for Organ Transplants: APAC is experiencing a demographic shift, with the population aged 60 and above increasing rapidly. By 2024, Japan will have over 36 million individuals aged 65 and above, making up nearly 30% of the population, according to the United Nations. This aging population is driving the demand for organ transplants, tissue regeneration, and age-related therapies such as stem cell treatments. Countries like South Korea and Singapore are experiencing similar trends, where age-related degenerative diseases are prompting an increased focus on regenerative medicine solutions to address healthcare needs.
APAC Regenerative Medicine Market Restraints
- Regulatory and Ethical Hurdles: Regenerative medicine faces numerous regulatory challenges in APAC, with varying degrees of stringency across countries. For instance, in 2022, China implemented more stringent rules on stem cell research and clinical trials, requiring multi-layered approvals from both provincial and national regulatory bodies. These complex regulations often delay the approval process for new therapies. Ethical concerns also arise, particularly regarding the use of embryonic stem cells, which face opposition from certain religious and cultural groups in the region.
- Lack of Standardization in Regenerative Medicine: The lack of standardization in regenerative medicine across the APAC region poses a significant challenge. Countries like India and Indonesia lack a unified framework for clinical trials, manufacturing, and the commercialization of regenerative therapies. In contrast, Japan and South Korea have established stringent regulatory systems, making it difficult for companies to navigate cross-border compliance. This fragmented regulatory environment hinders the consistent delivery of safe and effective therapies across the region, delaying market growth.
APAC Regenerative Medicine Market Future Outlook
Over the next five years, the APAC regenerative medicine market is expected to witness substantial growth, driven by government policies favoring advanced biotechnology, increasing investment in R&D, and the rising demand for innovative therapeutic solutions. The integration of artificial intelligence and 3D bioprinting technologies is set to enhance the capabilities of regenerative medicine, particularly in tissue engineering and gene therapy applications. Countries like China and Japan will likely continue leading the charge due to their strategic focus on healthcare innovation and research.
Market Opportunities
- Technological Advancements in Tissue Engineering: Technological advancements in tissue engineering are creating new growth opportunities for the APAC regenerative medicine market. In 2023, Singapore's A*STAR research agency developed a breakthrough 3D tissue scaffold capable of regenerating damaged heart tissue, opening new avenues for cardiac care. Similarly, Japans Kyoto University achieved significant progress in the development of tissue-engineered skin for burn victims. These technological innovations are expected to drive adoption across various therapeutic areas, providing enhanced treatment options for patients suffering from organ damage.
- Expansion of Clinical Applications: The APAC region is witnessing an expansion in the clinical applications of regenerative therapies. In 2023, South Korea saw the approval of the worlds first stem cell therapy for severe atopic dermatitis, marking a major milestone for regenerative medicine. Meanwhile, Australia is rapidly advancing in regenerative ophthalmology, with stem cell-based corneal transplants becoming more prevalent in clinical practice. These expanded applications highlight the therapeutic versatility of regenerative medicine, driving future market growth.
Scope of the Report
|
Therapy Type |
Cell Therapy Gene Therapy Tissue Engineering Immunotherapy |
|
Application |
Orthopedic Oncology Cardiovascular Dermatology Others (Neurological, Ophthalmology) |
|
Material Type |
Biologically Derived Materials Synthetic Materials Genetically Engineered Materials Scaffolding Materials |
|
End-User |
Hospitals and Clinics Academic & Research Institutes Biotech & Pharma Companies Ambulatory Surgical Centers |
|
Country |
China Japan South Korea India Australia |
Products
Key Target Audience
Investors and Venture Capitalist Firms
Biotech and Pharmaceutical Companies
Government and Regulatory Bodies (Ministry of Health, Japan's PMDA, China's NMPA)
Hospitals and Clinics
Academic & Research Institutes
Stem Cell and Gene Therapy Research Labs
Healthcare Providers
Biomanufacturing Facilities
Time Period Captured in the Report:
Historical Period: 2018-2023
Base Year: 2023
Forecast Period: 2023-2028
Companies
Players Mentioned in the Report:
JCR Pharmaceuticals Co., Ltd.
Fujifilm Holdings Corporation
Mesoblast Limited
Astellas Pharma Inc.
Kolon TissueGene, Inc.
Hitachi Chemical Co., Ltd.
Takeda Pharmaceutical Company Limited
Samsung Biologics
Lonza Group
Cytori Therapeutics Inc.
Table of Contents
1. APAC Regenerative Medicine Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. APAC Regenerative Medicine Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. APAC Regenerative Medicine Market Analysis
3.1.Growth Drivers
3.1.1. Rising Prevalence of Chronic Diseases
3.1.2. Increased Government Funding for Regenerative Medicine
3.1.3. Aging Population and Rising Demand for Organ Transplants
3.1.4. Growing Adoption of Stem Cell Technologies
3.2. Market Challenges
3.2.1. High Cost of Regenerative Therapies
3.2.2. Regulatory and Ethical Hurdles (Government Regulations)
3.2.3. Lack of Standardization in Regenerative Medicine (Compliance)
3.2.4. Limited Skilled Workforce
3.3. Opportunities
3.3.1. Technological Advancements in Tissue Engineering
3.3.2. Expansion of Clinical Applications (Regenerative Therapies)
3.3.3. Collaborative Research Initiatives (Public-Private Partnerships)
3.4. Trends
3.4.1. Gene Therapy Integration
3.4.2. Development of 3D Bioprinting Technologies
3.4.3. Use of Artificial Intelligence in Regenerative Medicine
3.5. Government Regulations and Compliance
3.5.1. APAC-Specific Regulatory Policies (Clinical Trials & Approvals)
3.5.2. Guidelines on Stem Cell Research and Applications
3.5.3. Biosafety Standards
3.6. SWOT Analysis
3.6.1. Strengths
3.6.2. Weaknesses
3.6.3. Opportunities
3.6.4. Threats
3.7. Stakeholder Ecosystem (Industry Stakeholders, Regulators, Researchers)
3.8. Porters Five Forces Analysis
3.9. Competition Ecosystem
4. APAC Regenerative Medicine Market Segmentation
4.1. By Therapy Type (In Value %)
4.1.1. Cell Therapy
4.1.2. Gene Therapy
4.1.3. Tissue Engineering
4.1.4. Immunotherapy
4.2. By Application (In Value %)
4.2.1. Orthopedic Regenerative Medicine
4.2.2. Oncology Regenerative Medicine
4.2.3. Cardiovascular Regenerative Medicine
4.2.4. Dermatology Regenerative Medicine
4.2.5. Others (Neurological, Ophthalmology)
4.3. By Material Type (In Value %)
4.3.1. Biologically Derived Materials (Cells, Tissues)
4.3.2. Synthetic Materials (Biopolymers)
4.3.3. Genetically Engineered Materials
4.3.4. Scaffolding Materials
4.4. By End-User (In Value %)
4.4.1. Hospitals and Clinics
4.4.2. Academic & Research Institutes
4.4.3. Biotech & Pharma Companies
4.4.4. Ambulatory Surgical Centers
4.5. By Country (In Value %)
4.5.1. China
4.5.2. Japan
4.5.3. South Korea
4.5.4. India
4.5.5. Australia
4.5.6. Rest of APAC
5. APAC Regenerative Medicine Market Competitive Analysis
5.1.Detailed Profiles of Major Companies
5.1.1. JCR Pharmaceuticals Co., Ltd.
5.1.2. Fujifilm Holdings Corporation
5.1.3. Mesoblast Limited
5.1.4. Astellas Pharma Inc.
5.1.5. Kolon TissueGene, Inc.
5.1.6. Hitachi Chemical Co., Ltd.
5.1.7. Takeda Pharmaceutical Company Limited
5.1.8. Samsung Biologics
5.1.9. Lonza Group
5.1.10. Cytori Therapeutics Inc.
5.1.11. Medipost Co., Ltd.
5.1.12. Pharmicell Co., Ltd.
5.1.13. GenScript Biotech Corporation
5.1.14. Pluristem Therapeutics Inc.
5.1.15. Cellular Biomedicine Group, Inc.
5.2. Cross Comparison Parameters (Market Share, Number of Patents, R&D Spending, Regional Presence, Strategic Alliances, Product Pipeline, Manufacturing Capacity, Regulatory Approvals)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Government Funding
5.8. Venture Capital Investments
5.9. Collaborative Partnerships
6. APAC Regenerative Medicine Market Regulatory Framework
6.1. Regulatory Guidelines for Stem Cell Therapy
6.2. Gene Therapy Approval Process
6.3. Tissue Engineering Regulations
6.4. Compliance Requirements for Biologics Manufacturing
7. APAC Regenerative Medicine Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. APAC Regenerative Medicine Future Market Segmentation
8.1. By Therapy Type (In Value %)
8.2. By Application (In Value %)
8.3. By Material Type (In Value %)
8.4. By End-User (In Value %)
8.5. By Country (In Value %)
9. APAC Regenerative Medicine Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Market Positioning Strategy
9.3. Customer Acquisition and Retention Strategies
9.4. White Space Opportunity Analysis
Disclaimer
Contact Us
Research Methodology
Step 1: Identification of Key Variables
The first step involved mapping out the entire regenerative medicine ecosystem in the APAC region. This step included extensive desk research from both secondary and proprietary databases to identify key variables influencing the market, such as technology adoption rates, clinical trial approvals, and regulatory landscape.
Step 2: Market Analysis and Construction
Historical data on regenerative medicine, including adoption trends in cell and gene therapies, were compiled to assess market penetration and growth. The analysis focused on therapy types and their revenue contributions in different countries across APAC.
Step 3: Hypothesis Validation and Expert Consultation
Hypotheses about market growth and challenges were validated through interviews with experts from the regenerative medicine field. These consultations provided valuable insights into market trends, product pipelines, and operational challenges faced by key market players.
Step 4: Research Synthesis and Final Output
The final stage involved synthesizing data gathered from industry reports, interviews, and database research to produce an accurate and validated market forecast. This approach ensured that the analysis captures the complexity and growth potential of the APAC regenerative medicine market.
Frequently Asked Questions
01. How big is the APAC Regenerative Medicine Market?
The APAC regenerative medicine market size by revenue USD 3.5 billion, driven by increasing investments in biotech research, government initiatives, and the rising need for advanced therapeutic solutions.
02. What are the challenges in the APAC Regenerative Medicine Market?
Key challenges include high costs of therapy, regulatory hurdles, and the lack of standardized guidelines across countries, which can hinder market penetration and slow clinical adoption.
03. Who are the major players in the APAC Regenerative Medicine Market?
Major players include JCR Pharmaceuticals, Fujifilm Holdings, Mesoblast Limited, and Kolon TissueGene. These companies dominate through technological advancements and strategic partnerships in the field.
04. What are the growth drivers of the APAC Regenerative Medicine Market?
The market is driven by the increasing prevalence of chronic diseases, advancements in stem cell therapies, and rising investments in biotechnological innovations, particularly in China and Japan.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















