
Asia Pacific Albumin Market Outlook to 2030
Region:Asia
Author(s):Naman Rohilla
Product Code:KROD8705
December 2024
94
About the Report
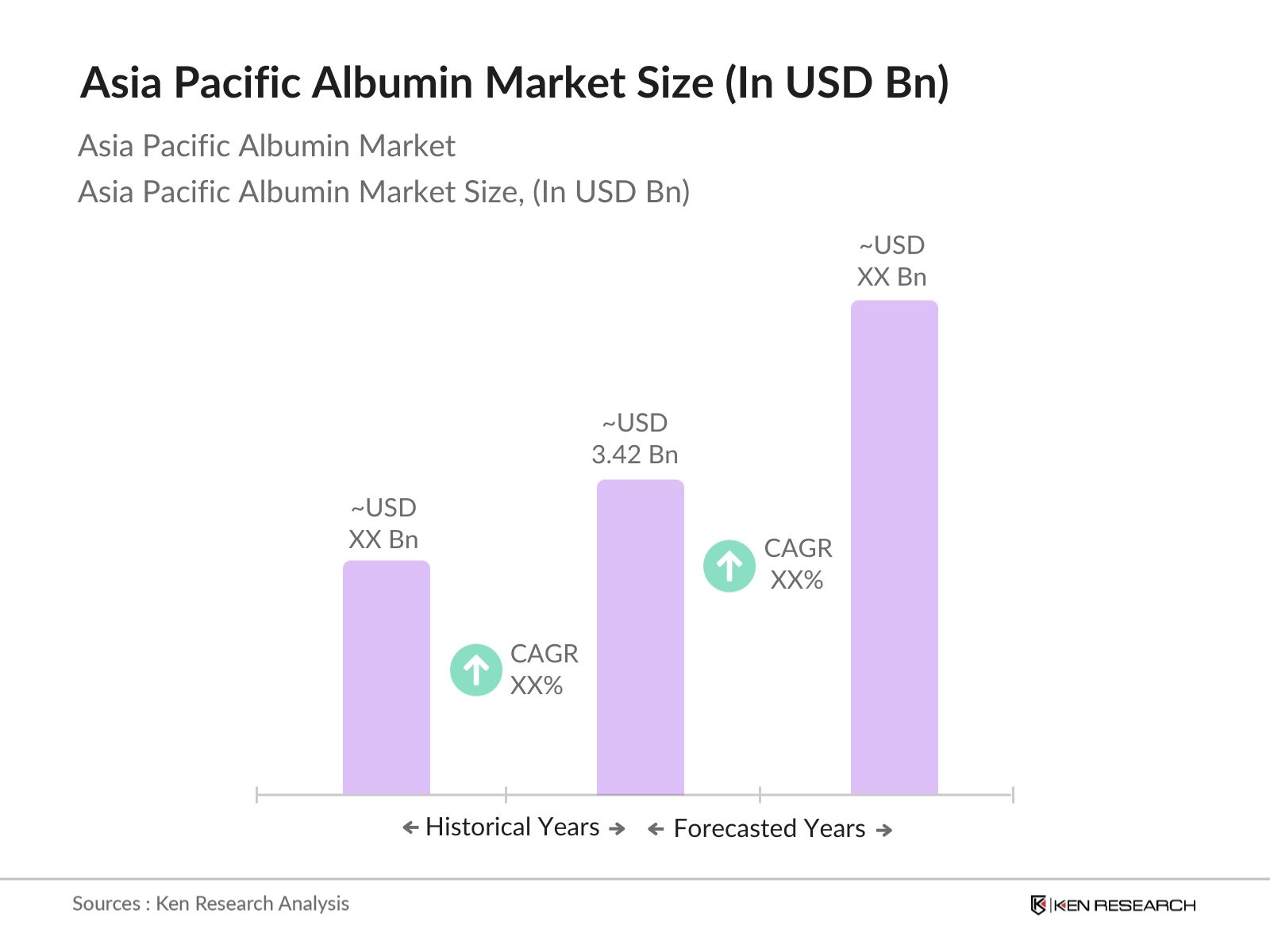
Asia Pacific Albumin Market Overview
- The Asia Pacific Albumin Market is valued at USD 3.42 billion, driven by increasing demand in biopharmaceuticals and the expanding plasma fractionation industry. This valuation reflects the cumulative efforts in research advancements and the widespread adoption of albumin in therapeutic treatments, particularly in immune-deficient and chronic disease patients. These factors underscore the steady expansion in the market, supported by major contributions from healthcare sectors across major economies.
- Countries such as China, Japan, and India dominate the Asia Pacific Albumin Market due to their advanced biopharmaceutical infrastructure and rising healthcare investments. China leads as a key player owing to large-scale production facilities and government-backed research funding. Japan and India contribute substantially, supported by regulatory frameworks that favor biotechnology expansion, making these nations critical to the markets growth dynamics.
- Asia-Pacific countries have established rigorous compliance standards for albumin production, focusing on quality and safety measures. In 2024, the National Health Regulatory Authority in India enforced stricter compliance norms, requiring bi-annual facility audits and comprehensive testing protocols. These regulations ensure high-quality albumin products in the region, protecting consumers while adding compliance costs for producers.
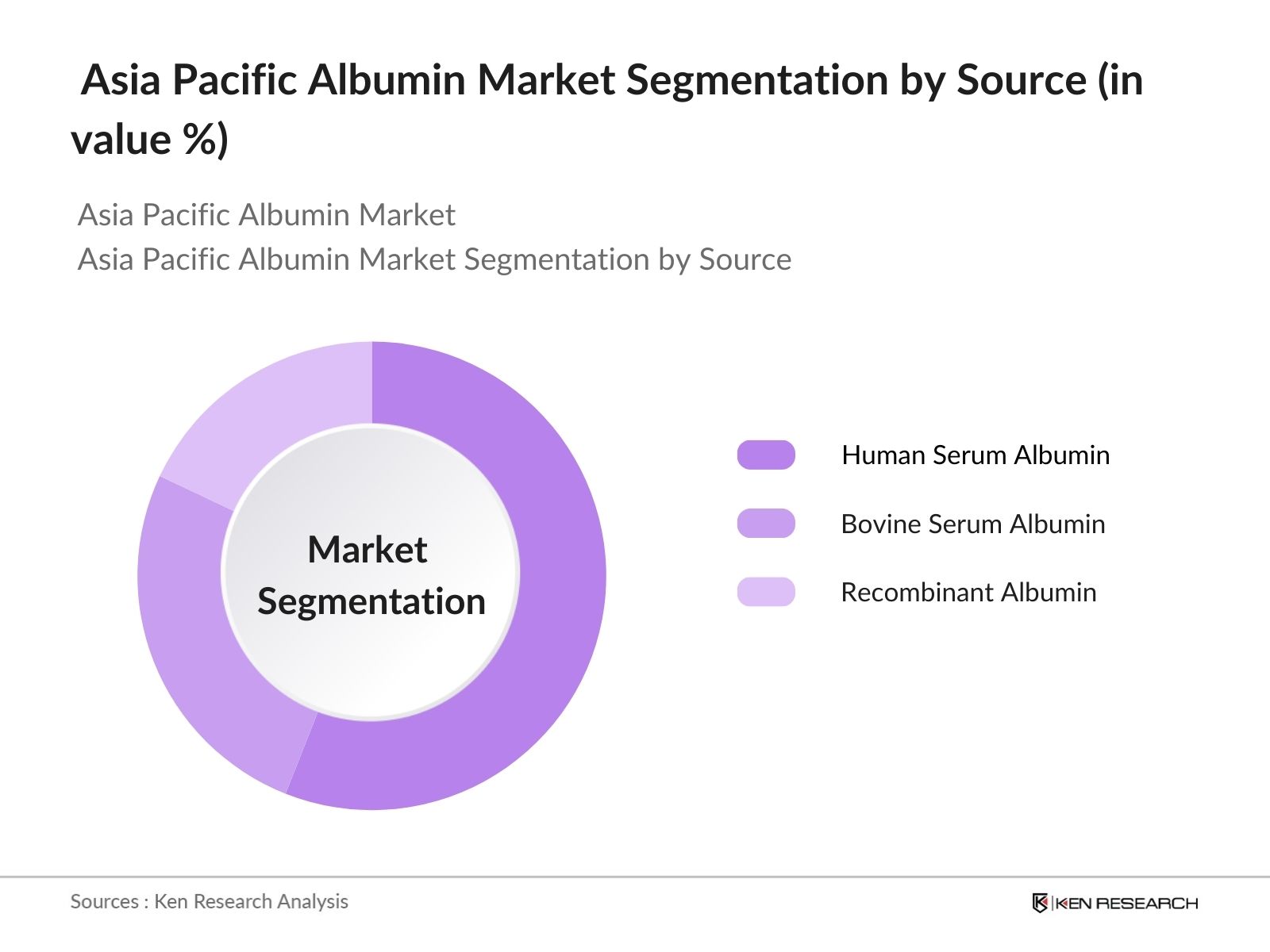
Asia Pacific Albumin Market Segmentation
- By Source: The market is segmented by source into Human Serum Albumin, Bovine Serum Albumin, and Recombinant Albumin. Recently, Human Serum Albumin has a dominant market share within this segmentation, attributed to its high efficacy in therapeutic applications, especially for patients with liver disease and hypovolemia. Its widespread adoption in these applications highlights its critical role in clinical settings, driving its prevalence within the market.
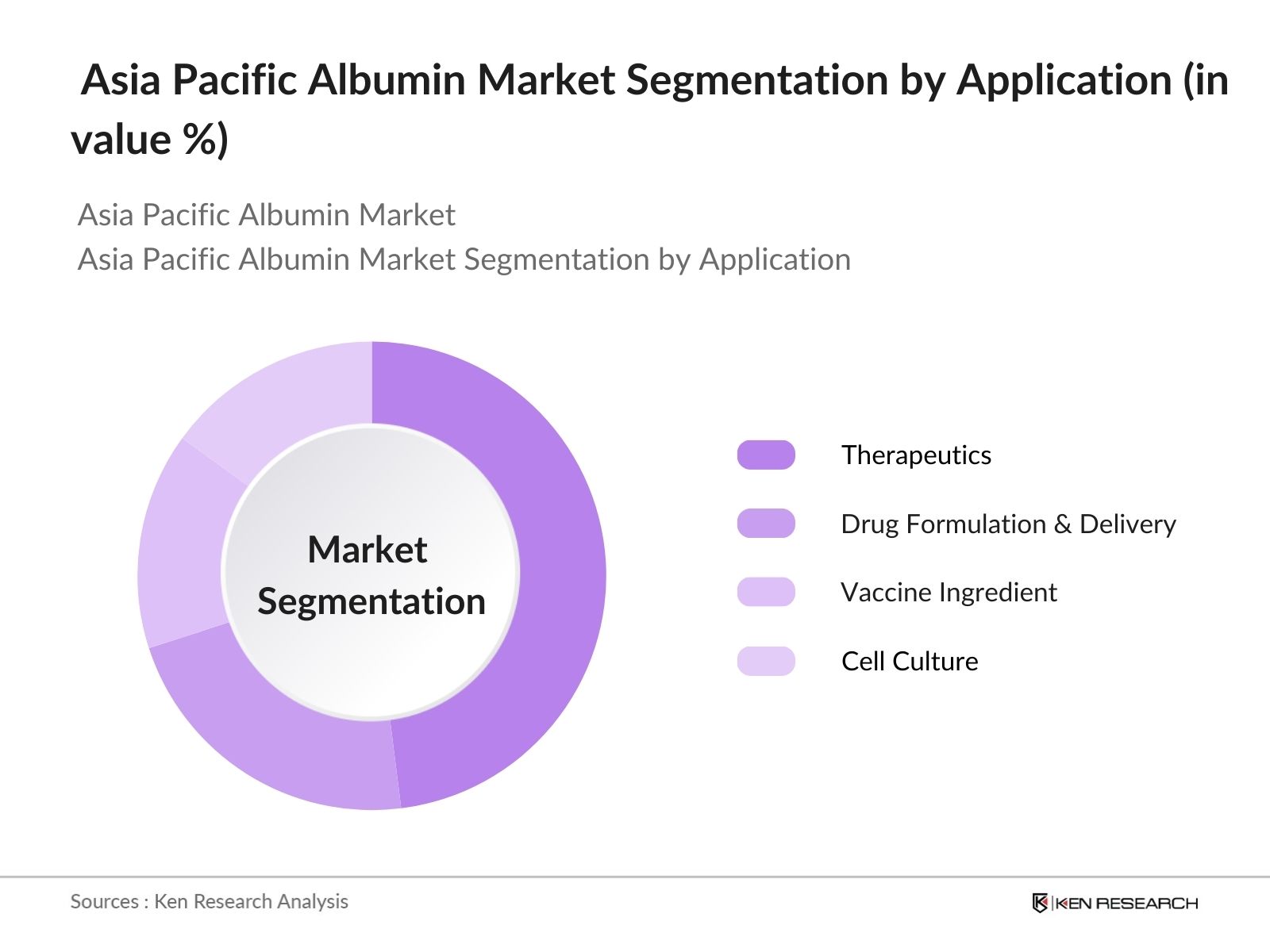
- By Application: The market is further segmented by application into Therapeutics, Drug Formulation & Drug Delivery, Vaccine Ingredient, and Cell Culture. Within this segmentation, Therapeutics holds a notable market share, primarily due to the critical role of albumin in addressing protein deficiency and managing critical health conditions. Its therapeutic uses in restoring blood volume and enhancing immune responses contribute substantially to its high market presence.
Asia Pacific Albumin Market Competitive Landscape
The Asia Pacific Albumin Market is led by a handful of established players with extensive market reach and competitive strengths. The competitive landscape is consolidated, with key players leveraging their robust R&D capabilities and extensive distribution networks to maintain market dominance. Companies like CSL Behring, Grifols S.A., and Takeda Pharmaceuticals are some of the notable participants, each contributing to innovation and market share.
Company | Establishment Year | Headquarters | Market Presence | R&D Investment | Product Portfolio | Market Expansion Efforts | Collaborations |
CSL Behring | 1916 | Melbourne, Australia | - | - | - | - | - |
Grifols S.A. | 1940 | Barcelona, Spain | - | - | - | - | - |
Takeda Pharmaceuticals | 1781 | Tokyo, Japan | - | - | - | - | - |
Octapharma AG | 1983 | Lachen, Switzerland | - | - | - | - | - |
Hualan Biological | 1992 | Xinxiang, China | - | - | - | - | - |
Asia Pacific Albumin Market Analysis
Market Growth Drivers
- Demand in Biopharmaceuticals: The Asia-Pacific biopharmaceutical sector is experiencing growth due to rising healthcare needs across an aging population, contributing to a higher demand for albumin. In 2024, the Asia-Pacific region, led by China and Japan, accounted for substantial investments in biopharmaceuticals, with China investing around USD 52 billion in healthcare advancements, reflecting increased albumin demand for therapeutic uses such as liver disease and hypoalbuminemia treatments. Albumins applications in stabilizing biopharmaceutical products like vaccines and therapeutics are supported by governmental funding in these countries, enhancing the markets expansion.
- Plasma Fractionation Expansion: Plasma fractionation processes, essential for albumin extraction, are expanding in the Asia-Pacific region, with substantial government support for local production capabilities. Japan and South Korea have invested in modernizing plasma fractionation infrastructure; Japan allocated USD 1.5 billion in 2023 to streamline these facilities and enhance plasma-based product availability. These advancements bolster albumin production, facilitating its availability across various therapeutic fields, addressing regional healthcare demands and reducing import dependence.
- Aging Population and Associated Health Conditions: An aging population across the Asia-Pacific region has led to increased incidences of albumin-necessitating health conditions like hypoalbuminemia. Japan, where 28% of the population is over 65, and South Korea, with a rapidly aging demographic, have noted a marked rise in elderly care requirements, bolstering the demand for albumin-based treatments. The World Bank reported that healthcare expenditures in these nations have surged, with Japans healthcare spending reaching USD 540 billion, reflecting an upward trajectory in demand for therapeutic albumin.
Market Challenges
- Supply Constraints: The Asia-Pacific region faces challenges in meeting albumin demand due to supply constraints, particularly in countries dependent on imports. The Philippines and Indonesia have reported limited local production, relying heavily on imports from leading suppliers like Japan and South Korea. Supply chain disruptions in recent years have exacerbated this issue, with Indonesias albumin import reliance constituting 78% of its total supply in 2023, leading to price volatility and accessibility issues.
- Stringent Regulatory Requirements: Compliance with stringent regulatory frameworks has posed challenges for albumin production and distribution within Asia-Pacific markets. Countries like China and India have stringent policies for plasma-derived products, requiring intensive approval processes and quality compliance. The Indian National Regulatory Authority reported that companies need an average of two years to obtain full licensure for albumin production due to rigorous inspection and validation processes, slowing market expansion.
Asia Pacific Albumin Market Future Outlook
The Asia Pacific Albumin Market is projected to witness substantial growth over the upcoming years, driven by innovations in plasma fractionation technologies, increasing therapeutic applications, and supportive regulatory frameworks. Growth factors include the development of synthetic albumin and advancements in recombinant technologies that enhance application efficiency across various therapeutic domains. The rising demand for albumin-based drugs will continue to propel market expansion, supported by sustained investments in research and development.
Market Opportunities
- Synthetic Alternatives Development: Synthetic alternatives to human-derived albumin are emerging as a viable solution, reducing dependency on blood donations and providing more consistent supply options. Japans RIKEN Research Institute invested USD 180 million in 2023 to accelerate synthetic albumin development, creating a sustainable pathway for albumin production and reducing risks linked to donor supply variability. This investment is expected to boost the regions self-sufficiency in albumin production without relying on plasma donation.
- Technological Innovations in Albumin Extraction: Innovations in albumin extraction, such as advancements in recombinant technologies, are enhancing efficiency in albumin production. South Koreas biotech sector has introduced high-yield extraction processes, reducing production time and environmental impact. In 2023, the South Korean Ministry of Health allocated USD 120 million toward biotechnology innovations to improve albumin extraction efficiency, reflecting the potential to increase production rates while reducing dependency on traditional fractionation methods.
Scope of the Report
Source | Human Serum Albumin |
Application | Therapeutics |
End-User | Hospitals and Clinics |
Grade | Technical Grade |
Country | China |
Products
Key Target Audience
Pharmaceutical Manufacturers
Biopharmaceutical Companies
Contract Research Organizations (CROs)
Plasma Fractionation Service Providers
Research Institutes and Universities
Banks and Financial Institutions
Investor and Venture Capitalist Firms
Government and Regulatory Bodies (FDA, CFDA, TGA)
Healthcare Providers and Clinical Facilities
Companies
Players Mentioned in the Report
CSL Behring
Grifols S.A.
Takeda Pharmaceuticals
Octapharma AG
Hualan Biological Engineering Inc.
Baxter International Inc.
Biotest AG
Sanquin Blood Supply Foundation
Kedrion S.p.A
LFB Group
Table of Contents
1. Asia Pacific Albumin Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy
1.3 Market Growth Rate
1.4 Market Segmentation Overview
2. Asia Pacific Albumin Market Size (in USD Bn)
2.1 Historical Market Size
2.2 Year-On-Year Growth Analysis
2.3 Key Market Developments and Milestones
3. Asia Pacific Albumin Market Analysis
3.1 Growth Drivers
3.1.1 Demand in Biopharmaceuticals
3.1.2 Plasma Fractionation Expansion
3.1.3 Aging Population and Associated Health Conditions
3.1.4 R&D Advancements in Albumin Applications
3.2 Market Challenges
3.2.1 Supply Constraints
3.2.2 Stringent Regulatory Requirements
3.2.3 High Costs of Albumin Production
3.2.4 Market Dependence on Blood Donations
3.3 Opportunities
3.3.1 Synthetic Alternatives Development
3.3.2 Technological Innovations in Albumin Extraction
3.3.3 Expansion into Cosmetic and Food Industries
3.4 Trends
3.4.1 Growing Preference for Recombinant Albumin
3.4.2 Increased Applications in Regenerative Medicine
3.4.3 Sustainable Sourcing Initiatives
3.5 Regulatory Framework
3.5.1 Compliance Standards
3.5.2 Licensing and Certification Requirements
3.5.3 Ethical Sourcing and Production Guidelines
3.6 SWOT Analysis
3.7 Stakeholder Ecosystem
3.8 Porters Five Forces Analysis
3.9 Competitive Ecosystem
4. Asia Pacific Albumin Market Segmentation
4.1 By Source (In Value %)
4.1.1 Human Serum Albumin
4.1.2 Bovine Serum Albumin
4.1.3 Recombinant Albumin
4.2 By Application (In Value %)
4.2.1 Therapeutics
4.2.2 Drug Formulation & Drug Delivery
4.2.3 Vaccine Ingredient
4.2.4 Cell Culture
4.3 By End-User (In Value %)
4.3.1 Hospitals and Clinics
4.3.2 Research Institutes
4.3.3 Biopharmaceutical Companies
4.4 By Grade (In Value %)
4.4.1 Technical Grade
4.4.2 Food Grade
4.4.3 Pharmaceutical Grade
4.5 By Country (In Value %)
4.5.1 China
4.5.2 Japan
4.5.3 India
4.5.4 South Korea
4.5.5 Australia
5. Asia Pacific Albumin Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1 CSL Behring
5.1.2 Grifols S.A.
5.1.3 Takeda Pharmaceuticals
5.1.4 Octapharma AG
5.1.5 Baxter International Inc.
5.1.6 Biotest AG
5.1.7 Sanquin Blood Supply Foundation
5.1.8 Hualan Biological Engineering Inc.
5.1.9 Kedrion S.p.A
5.1.10 LFB Group
5.1.11 Green Cross Corporation
5.1.12 Shanghai RAAS Blood Products Co., Ltd
5.1.13 China Biologic Products Holdings, Inc.
5.1.14 Shire PLC
5.1.15 Bio Products Laboratory Ltd
5.2 Cross Comparison Parameters (Market Share %, Revenue, Product Portfolio, Innovation Capability, Distribution Network, Regulatory Compliance, Manufacturing Capacity, Brand Reputation)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers and Acquisitions
5.6 Investment Analysis
5.7 Joint Ventures and Partnerships
5.8 R&D Expenditure
5.9 Market Positioning
6. Asia Pacific Albumin Market Regulatory Framework
6.1 Regional Compliance Standards
6.2 Import/Export Regulations
6.3 Quality Control and Certification Requirements
7. Asia Pacific Albumin Future Market Size (in USD Bn)
7.1 Projected Market Growth
7.2 Key Future Growth Drivers
8. Asia Pacific Albumin Future Market Segmentation
8.1 By Source (In Value %)
8.2 By Application (In Value %)
8.3 By End-User (In Value %)
8.4 By Grade (In Value %)
8.5 By Country (In Value %)
9. Asia Pacific Albumin Market Analysts Recommendations
9.1 Market Entry Strategies
9.2 Product Diversification Opportunities
9.3 Value Chain Optimization
9.4 White Space Opportunities
DisclaimerContact UsResearch Methodology
Step 1: Identification of Key Variables
The research process began with constructing a comprehensive ecosystem map to include all stakeholders within the Asia Pacific Albumin Market. Through extensive desk research, we identified key variables that influence market dynamics, particularly focusing on production trends, regulatory frameworks, and demand drivers.
Step 2: Market Analysis and Data Compilation
In this phase, we gathered and analyzed historical data, assessing the role of various factors on market revenue generation. Emphasis was placed on quality control standards, plasma fractionation efficiency, and the resulting product distribution patterns to accurately establish baseline market insights.
Step 3: Hypothesis Validation and Expert Consultation
Hypotheses on market trends and growth drivers were validated through expert interviews and consultations. Insights from professionals in biopharmaceutical companies and research institutes were incorporated to confirm the projected market direction and emerging challenges.
Step 4: Synthesis and Final Report Preparation
The final phase involved synthesizing insights from secondary and primary research. This was supported by cross-validation techniques to enhance data reliability, ensuring that each reported statistic and trend accurately reflects the Asia Pacific Albumin Market's current state.
Frequently Asked Questions
01. How big is the Asia Pacific Albumin Market?
The Asia Pacific Albumin Market is valued at USD 3.42 billion, with its growth propelled by increasing therapeutic demand and expansion in plasma fractionation technologies.
02. What are the primary challenges in the Asia Pacific Albumin Market?
Key challenges include regulatory compliance, high production costs, and supply limitations, as the market heavily relies on donations for raw materials.
03. Who are the major players in the Asia Pacific Albumin Market?
Leading companies include CSL Behring, Grifols S.A., and Takeda Pharmaceuticals, known for their extensive product portfolios, strong R&D investments, and established market presence.
04. What are the growth drivers of the Asia Pacific Albumin Market?
Growth drivers include the demand for albumin in biopharmaceutical applications, advancements in synthetic and recombinant albumin, and increasing investment in healthcare infrastructure across major countries.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















