
Asia Pacific Angioplasty Balloon Market Outlook to 2030
Region:Asia
Author(s):Meenakshi Bisht
Product Code:KROD5525
November 2024
88
About the Report
Asia Pacific Angioplasty Balloon Market Overview
- The Asia Pacific angioplasty balloon market, valued at USD 665 million, is primarily driven by the increasing prevalence of cardiovascular diseases and the growing adoption of minimally invasive procedures. Technological advancements in balloon design and materials have further propelled market growth, enhancing procedural efficacy and patient outcomes.
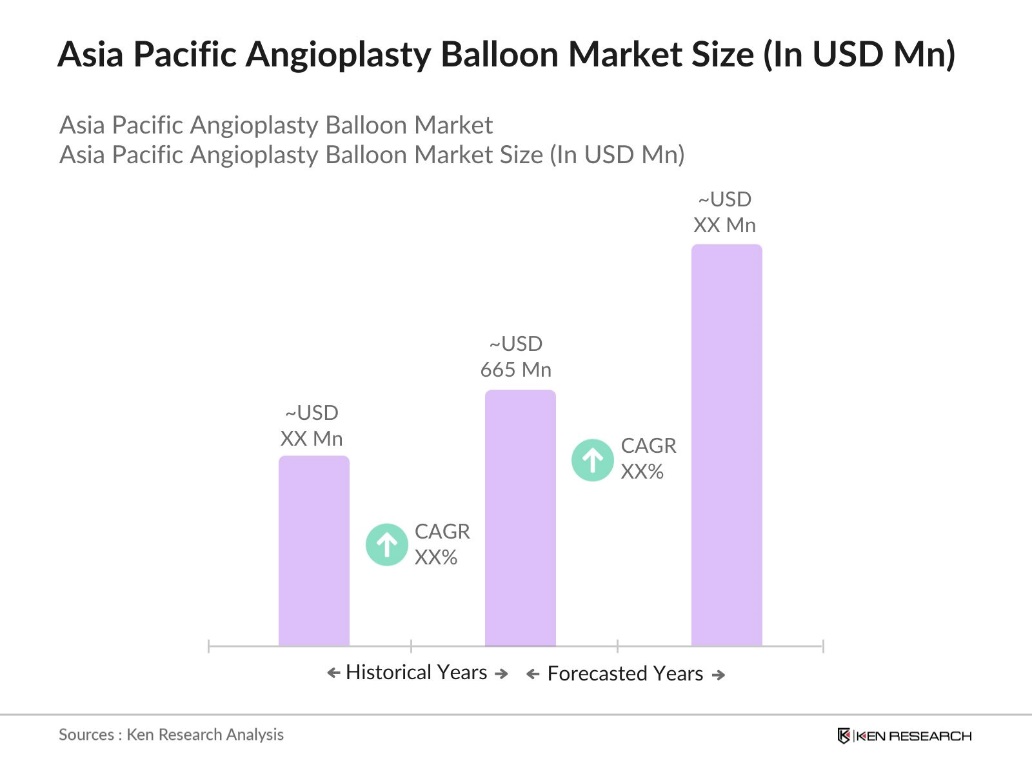
- China and Japan are the dominant countries in this market, attributed to their advanced healthcare infrastructures, high incidence of cardiovascular conditions, and substantial investments in medical technology. Additionally, the presence of leading medical device manufacturers in these nations contributes to their market leadership.
- Medical device regulations in Asia Pacific have become progressively stringent, aiming to align with international standards and ensure patient safety. In 2023, China introduced reforms under the National Medical Products Administration (NMPA) to simplify the approval process while upholding high safety standards, expediting market entry for critical devices. These evolving regulatory frameworks are vital for manufacturers seeking to enter the rapidly growing Asia Pacific market.
Asia Pacific Angioplasty Balloon Market Segmentation
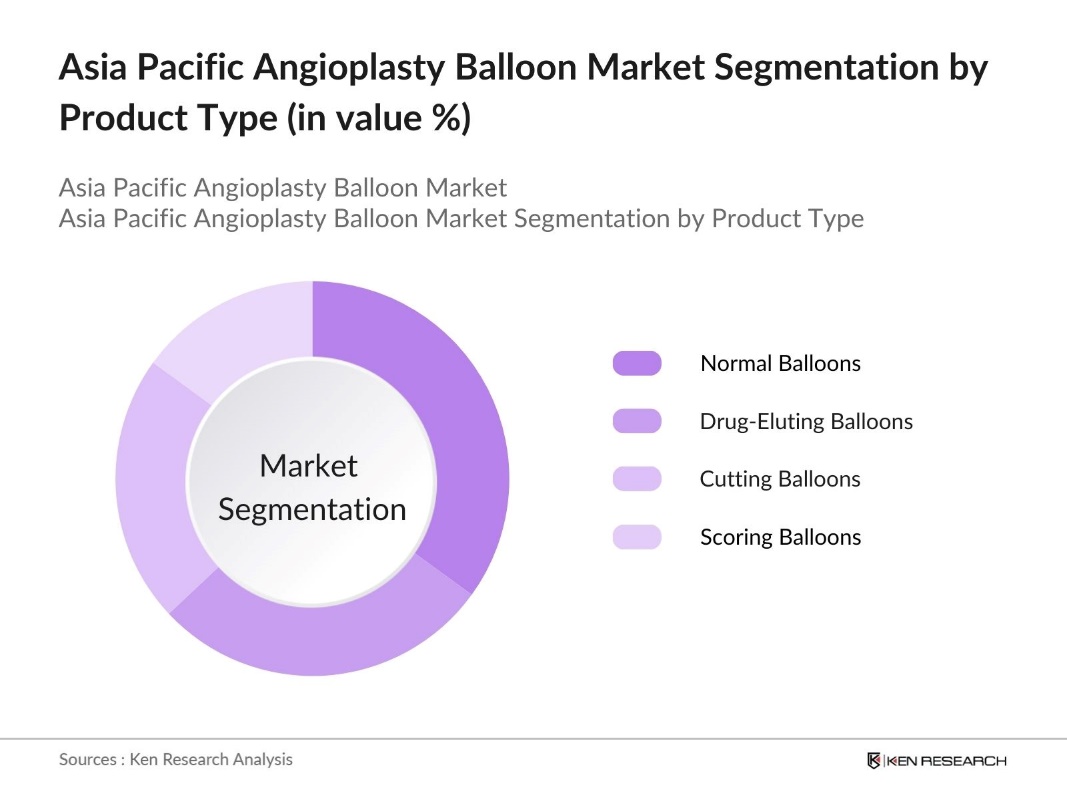
By Product Type: The market is segmented by product type into normal balloons, drug-eluting balloons, cutting balloons, and scoring balloons. Normal balloons hold a dominant market share due to their widespread use in standard angioplasty procedures and cost-effectiveness. Their established efficacy and availability make them a preferred choice among healthcare providers.
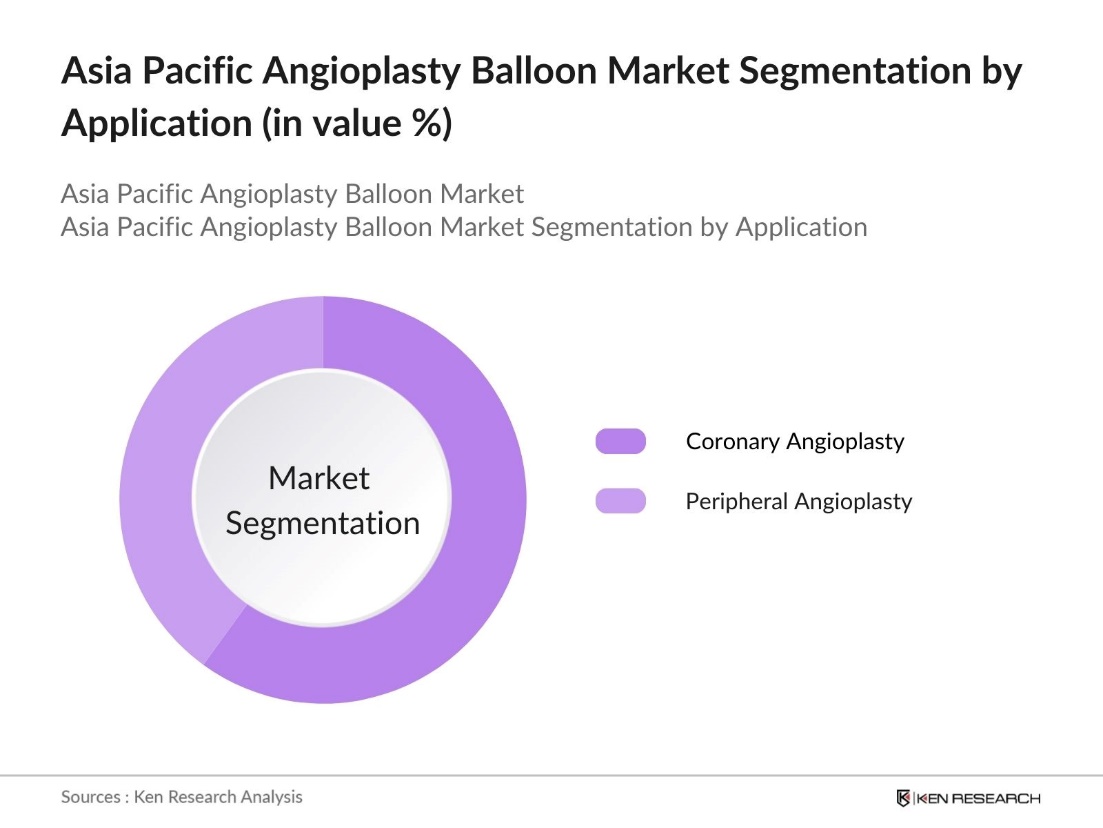
By Application: The market is segmented by application into coronary angioplasty and peripheral angioplasty. Coronary angioplasty dominates the market share, driven by the high prevalence of coronary artery diseases in the region. The increasing number of percutaneous coronary interventions contributes to the prominence of this segment.
Asia Pacific Angioplasty Balloon Market Competitive Landscape
The Asia Pacific angioplasty balloon market is characterized by the presence of several key players, including Abbott Laboratories, Medtronic plc, Boston Scientific Corporation, Terumo Corporation, and B. Braun Melsungen AG. These companies leverage their extensive product portfolios, strong distribution networks, and continuous innovation to maintain a competitive edge.
Asia Pacific Angioplasty Balloon Industry Analysis
Growth Drivers
- Increasing Prevalence of Cardiovascular Diseases: In 2022, CVD was responsible for approximately 19.8 million deaths worldwide, reflecting an increase from previous years due to factors such as population growth and aging, as well as preventable risk factors like high blood pressure and smoking. This escalating prevalence underscores the urgent need for effective interventions, thereby driving the demand for advanced medical devices, including specialized balloons used in minimally invasive procedures.
- Advancements in Minimally Invasive Procedures: Minimally invasive surgical techniques have transformed patient care by reducing recovery times and minimizing complications. In 2023, the total number of cosmetic minimally invasive procedures reached approximately 25.4 million, which represented a 7% increase from the previous year. Such advancements are propelling the demand for specialized medical devices, including balloons designed for these procedures.
- Technological Innovations in Balloon Design: Recent technological advancements have led to the development of drug-eluting balloons, which combine mechanical dilation with localized drug delivery to prevent restenosis. These innovations have shown promising results in clinical trials, offering improved patient outcomes. Additionally, the integration of imaging technologies with balloon catheters has enhanced procedural precision, further boosting their adoption in clinical settings.
Market Challenges
- Stringent Regulatory Approvals: The medical device industry is subject to rigorous regulatory standards to ensure patient safety and efficacy. Obtaining approval from bodies such as the U.S. Food and Drug Administration (FDA) or the European Medicines Agency (EMA) can be a lengthy and costly process. For example, the FDA's premarket approval process for high-risk devices can take several years and require substantial clinical data, posing challenges for manufacturers aiming to bring innovative balloon technologies to market promptly.
- Availability of Alternative Therapies: The presence of alternative treatments, such as pharmacological therapies and lifestyle interventions, can impact the demand for procedures involving specialized balloons. For instance, the use of statins and other cholesterol-lowering medications has been effective in managing certain cardiovascular conditions, potentially reducing the need for interventional procedures. Additionally, advancements in stent technologies offer alternative solutions for patients, presenting competition to balloon-based interventions.
Asia Pacific Angioplasty Balloon Market Future Outlook
Over the next five years, the Asia Pacific angioplasty balloon market is expected to experience significant growth, driven by continuous advancements in balloon technologies, increasing healthcare expenditure, and the rising prevalence of cardiovascular diseases. Emerging economies within the region are anticipated to offer lucrative opportunities for market expansion.
Market Opportunities
- Adoption of Drug-Eluting Balloons: Drug-eluting balloons (DEBs) have emerged as a promising technology in the treatment of vascular diseases. Clinical studies have demonstrated that DEBs can reduce restenosis rates and improve long-term patient outcomes compared to traditional angioplasty. The growing body of evidence supporting their efficacy is encouraging healthcare providers to adopt this technology, thereby creating new market opportunities.
- Government Initiatives for Healthcare Infrastructure: Governments worldwide are investing in healthcare infrastructure to improve access to quality care. For example, India's National Health Policy aims to increase public health expenditure to 2.5% of GDP by 2025, focusing on non-communicable diseases, including cardiovascular conditions. Such initiatives are expected to enhance the availability of advanced medical treatments, including procedures involving specialized balloons, thereby driving market growth.
Scope of the Report
|
Product Type |
Normal Balloons |
|
Application |
Coronary Angioplasty |
|
Material |
Nylon |
|
End User |
Hospitals |
|
Region |
China |
Products
Key Target Audience
Medical Device Manufacturers
Medical Equipment Leasing Firms
Healthcare Technology
Investors and Venture Capitalist Firms
Government and Regulatory Bodies (e.g., Ministry of Health)
Banks and Financial Institutions
Companies
Players Mentioned in the Report
Abbott Laboratories
Medtronic plc
Boston Scientific Corporation
Terumo Corporation
B. Braun Melsungen AG
BIOTRONIK SE & Co. KG
Cardinal Health, Inc.
Cook Medical LLC
Johnson & Johnson Services, Inc.
ENDOCOR GmbH
Table of Contents
1. Asia Pacific Angioplasty Balloon Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy
1.3 Market Growth Rate
1.4 Market Segmentation Overview
2. Asia Pacific Angioplasty Balloon Market Size (USD Mn)
2.1 Historical Market Size
2.2 Year-On-Year Growth Analysis
2.3 Key Market Developments and Milestones
3. Asia Pacific Angioplasty Balloon Market Analysis
3.1 Growth Drivers
3.1.1 Increasing Prevalence of Cardiovascular Diseases
3.1.2 Advancements in Minimally Invasive Procedures
3.1.3 Rising Geriatric Population
3.1.4 Technological Innovations in Balloon Design
3.2 Market Challenges
3.2.1 High Procedure Costs
3.2.2 Stringent Regulatory Approvals
3.2.3 Availability of Alternative Therapies
3.3 Opportunities
3.3.1 Emerging Markets in Asia Pacific
3.3.2 Adoption of Drug-Eluting Balloons
3.3.3 Government Initiatives for Healthcare Infrastructure
3.4 Trends
3.4.1 Integration of Imaging Technologies
3.4.2 Development of Biodegradable Balloons
3.4.3 Increasing Use of Cutting and Scoring Balloons
3.5 Government Regulations
3.5.1 Medical Device Regulations in Asia Pacific
3.5.2 Reimbursement Policies
3.5.3 Quality Standards and Compliance
3.6 SWOT Analysis
3.7 Stakeholder Ecosystem
3.8 Porters Five Forces Analysis
3.9 Competitive Landscape
4. Asia Pacific Angioplasty Balloon Market Segmentation
4.1 By Product Type (Value %)
4.1.1 Normal Balloons
4.1.2 Drug-Eluting Balloons
4.1.3 Cutting Balloons
4.1.4 Scoring Balloons
4.2 By Application (Value %)
4.2.1 Coronary Angioplasty
4.2.2 Peripheral Angioplasty
4.3 By Material (Value %)
4.3.1 Nylon
4.3.2 Polyurethane
4.3.3 Silicone Urethane Co-Polymers
4.4 By End User (Value %)
4.4.1 Hospitals
4.4.2 Ambulatory Surgical Centers
4.4.3 Specialty Clinics
4.5 By Region (Value %)
4.5.1 China
4.5.2 Japan
4.5.3 India
4.5.4 Australia
4.5.5 South Korea
4.5.6 Rest of Asia Pacific
5. Asia Pacific Angioplasty Balloon Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1 Abbott Laboratories
5.1.2 Medtronic plc
5.1.3 Boston Scientific Corporation
5.1.4 Terumo Corporation
5.1.5 B. Braun Melsungen AG
5.1.6 BIOTRONIK SE & Co. KG
5.1.7 Cardinal Health, Inc.
5.1.8 Cook Medical LLC
5.1.9 Johnson & Johnson Services, Inc.
5.1.10 ENDOCOR GmbH
5.1.11 C. R. Bard, Inc.
5.1.12 Spectranetics Corporation
5.1.13 Nipro Medical Corporation
5.1.14 Acrostak Int. Distr. Sarl
5.1.15 Hexacath International
5.2 Cross Comparison Parameters (Number of Employees, Headquarters, Inception Year, Revenue, Product Portfolio, Market Share, R&D Investment, Regional Presence)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers and Acquisitions
5.6 Investment Analysis
5.7 Venture Capital Funding
5.8 Government Grants
5.9 Private Equity Investments
6. Asia Pacific Angioplasty Balloon Market Regulatory Framework
6.1 Medical Device Regulations in Key Countries
6.2 Compliance Requirements
6.3 Certification Processes
7. Asia Pacific Angioplasty Balloon Future Market Size (USD Mn)
7.1 Future Market Size Projections
7.2 Key Factors Driving Future Market Growth
8. Asia Pacific Angioplasty Balloon Future Market Segmentation
8.1 By Product Type (Value %)
8.2 By Application (Value %)
8.3 By Material (Value %)
8.4 By End User (Value %)
8.5 By Region (Value %)
9. Asia Pacific Angioplasty Balloon Analysts Recommendations
9.1 Total Addressable Market (TAM), Serviceable Available Market (SAM), and Serviceable Obtainable Market (SOM) Analysis
9.2 Customer Cohort Analysis
9.3 Marketing Initiatives
9.4 White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The initial phase involves constructing an ecosystem map encompassing all major stakeholders within the Asia Pacific angioplasty balloon market. This step is underpinned by extensive desk research, utilizing a combination of secondary and proprietary databases to gather comprehensive industry-level information. The primary objective is to identify and define the critical variables that influence market dynamics.
Step 2: Market Analysis and Construction
In this phase, we compile and analyze historical data pertaining to the Asia Pacific angioplasty balloon market. This includes assessing market penetration, the ratio of marketplaces to service providers, and the resultant revenue generation. Furthermore, an evaluation of service quality statistics is conducted to ensure the reliability and accuracy of the revenue estimates.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are developed and subsequently validated through computer-assisted telephone interviews (CATIs) with industry experts representing a diverse array of companies. These consultations provide valuable operational and financial insights directly from industry practitioners, which are instrumental in refining and corroborating the market data.
Step 4: Research Synthesis and Final Output
The final phase involves direct engagement with multiple medical device manufacturers to acquire detailed insights into product segments, sales performance, consumer preferences, and other pertinent factors. This interaction serves to verify and complement the statistics derived from the bottom-up approach, thereby ensuring a comprehensive, accurate, and validated analysis of the Asia Pacific angioplasty balloon market.
Frequently Asked Questions
01 How big is the Asia Pacific angioplasty balloon market?
The Asia Pacific angioplasty balloon market is valued at USD 665 million, driven by the increasing prevalence of cardiovascular diseases and advancements in minimally invasive procedures.
02 What are the challenges in the Asia Pacific angioplasty balloon market?
Challenges in Asia Pacific angioplasty balloon market include high procedure costs, stringent regulatory approvals, and the availability of alternative therapies, which may hinder market growth.
03 Who are the major players in the Asia Pacific angioplasty balloon market?
Key players in the Asia Pacific angioplasty balloon market include Abbott Laboratories, Medtronic plc, Boston Scientific Corporation, Terumo Corporation, and B. Braun Melsungen AG, among others.
04 What are the growth drivers of the Asia Pacific angioplasty balloon market?
The Asia Pacific angioplasty balloon market is propelled by factors such as the increasing prevalence of cardiovascular diseases, advancements in balloon technologies, and the rising adoption of minimally invasive procedures.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















