
Asia-Pacific Cell Therapy Market Outlook to 2030
Region:Asia
Author(s):Naman Rohilla
Product Code:KROD2922
November 2024
89
About the Report
Asia-Pacific Cell Therapy Market Overview
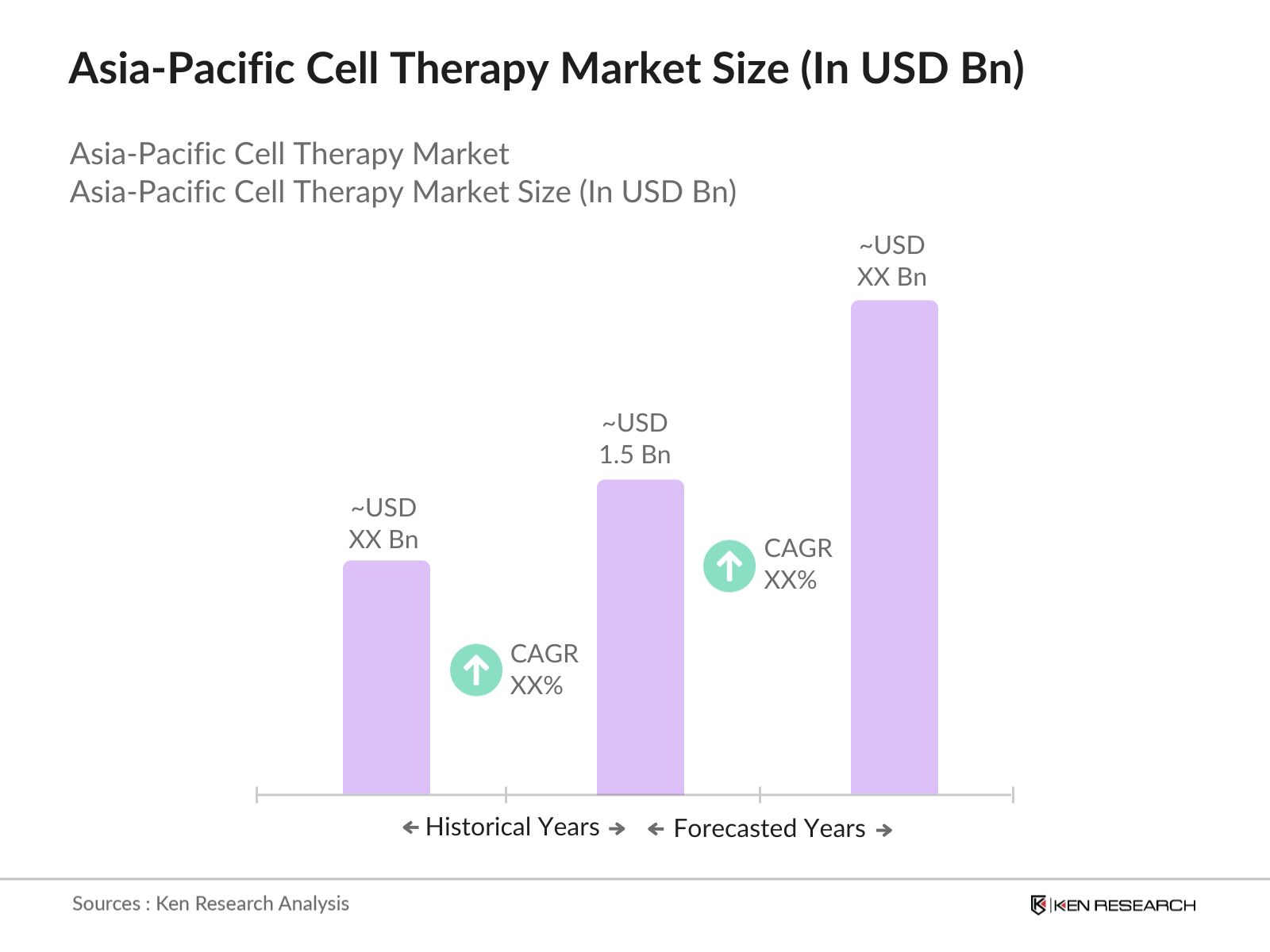
- The Asia Pacific cell therapy market is valued at USD 1.5 billion, driven by advancements in regenerative medicine and the increasing prevalence of chronic diseases like cancer and cardiovascular conditions. The rise in investments from both private firms and government bodies has fueled the growth of stem cell research and the development of novel cell therapies. Regulatory frameworks in countries like Japan and China have also played a pivotal role in boosting market expansion through initiatives like fast-track approval processes.
- Countries such as Japan, China, and South Korea dominate the cell therapy market in the Asia Pacific region. Japans leadership is driven by its robust regulatory support for regenerative medicine and well-established research infrastructure. China follows with strong government backing and a burgeoning biotechnology sector, while South Korea benefits from substantial investments in research and development. These countries provide the ideal ecosystem for rapid adoption and commercialization of cell therapies.
- The US FDAs regulatory framework for cell-based therapies influences Asia-Pacific markets, as many companies align their research and development efforts with FDA standards to facilitate future market entry into the United States. In 2023, several Asia-Pacific nations, including Singapore and South Korea, adopted FDA-inspired guidelines to streamline approval processes for cell therapies, ensuring their compatibility with global regulatory frameworks. This alignment is expected to enhance the region's attractiveness for international investors and clinical trials.
Asia-Pacific Cell Therapy Market Segmentation
The Asia-Pacific Cell Therapy Market is segmented by therapy type, application, cell type, end-users, and geographical region.
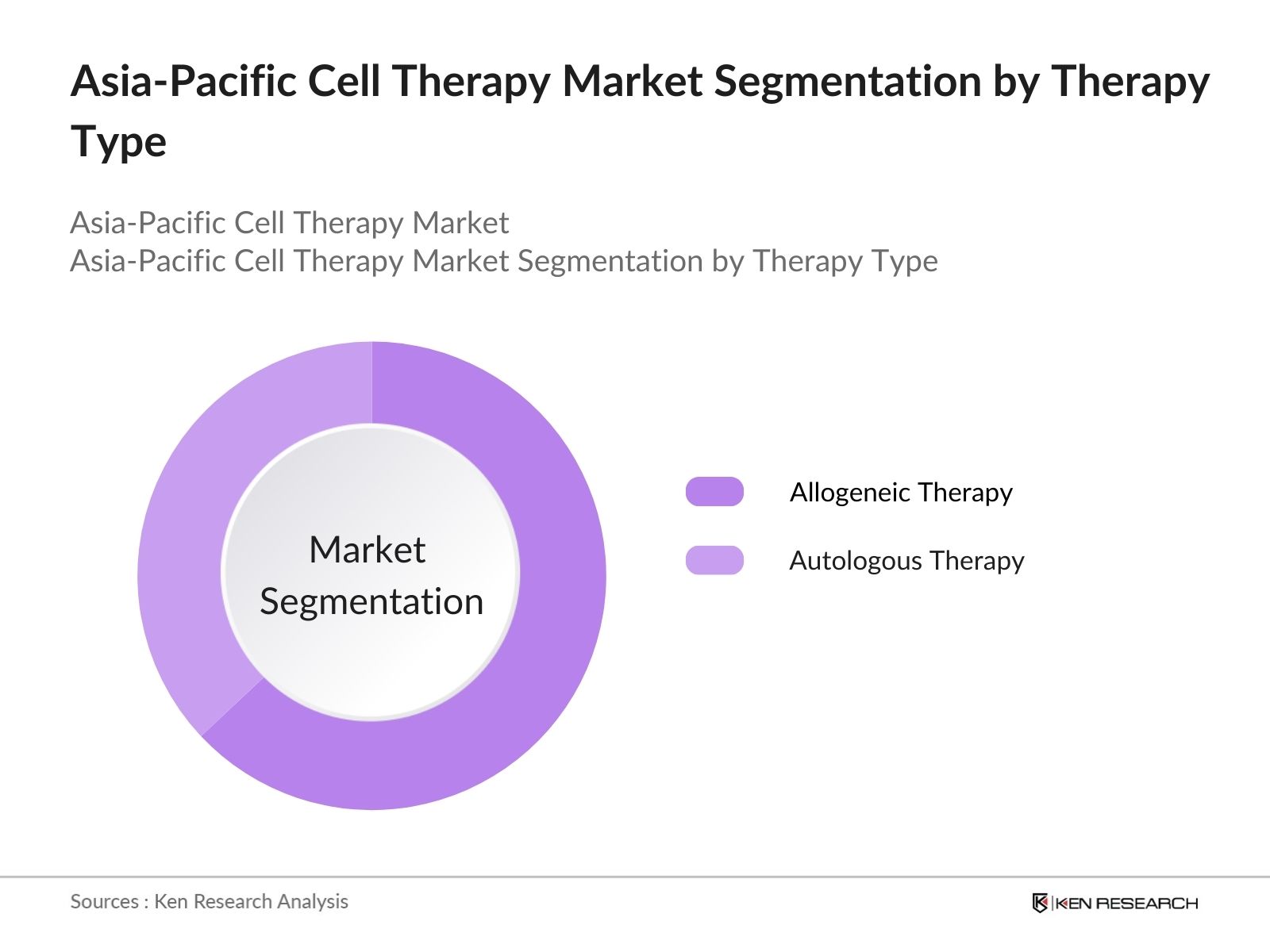
By Therapy Type: The Asia Pacific cell therapy market is segmented by therapy type into allogeneic therapy and autologous therapy. Allogeneic therapy has been the dominant market share due to its increasing adoption in treating complex conditions like cancer and neurodegenerative diseases. The wider availability of donor cells and the ability to mass-produce these therapies make them a more scalable option. Additionally, reduced risks of certain complications in allogeneic therapy have contributed to its growing popularity across clinical applications.
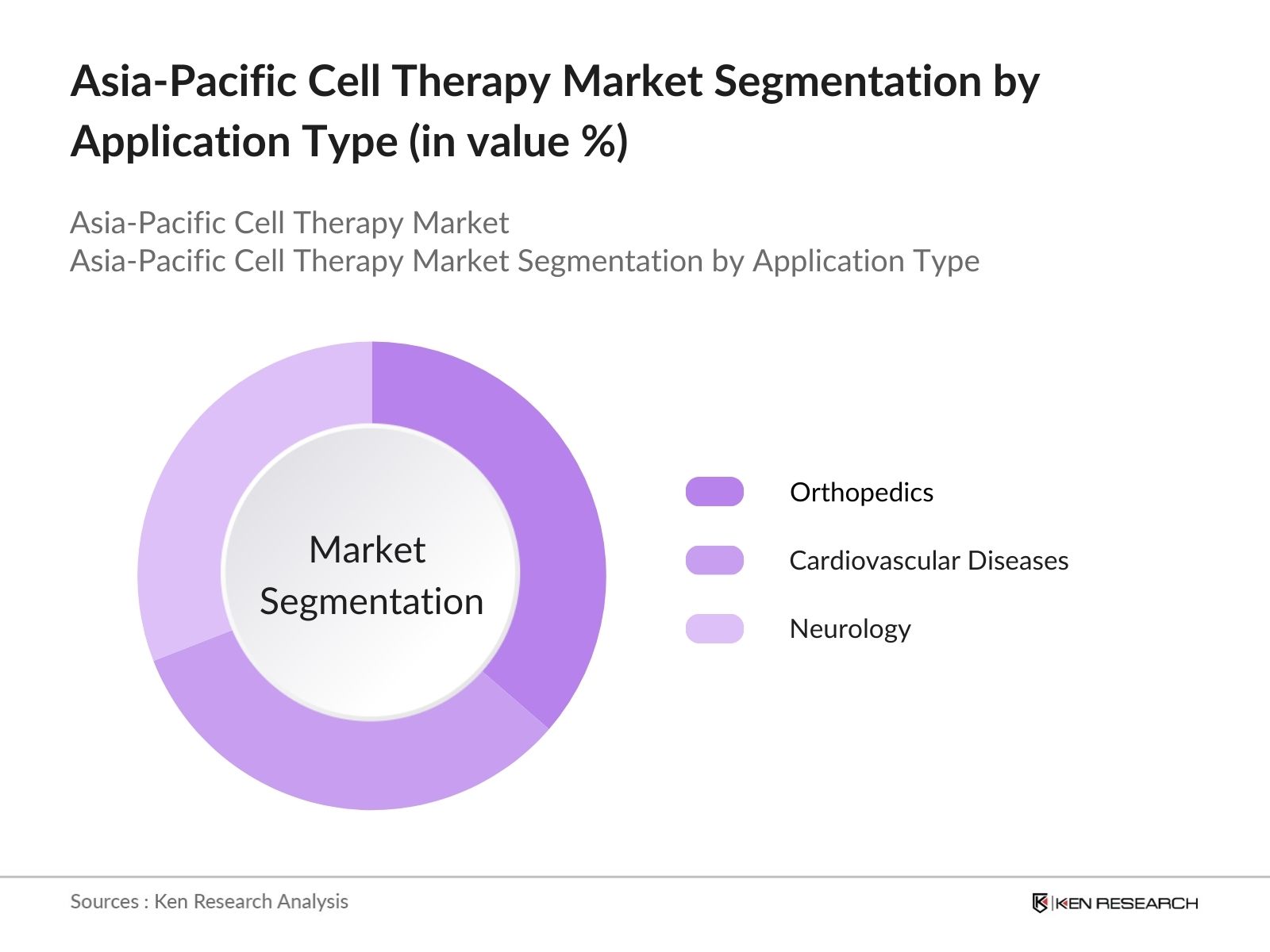
By Application: The market is also segmented by application into oncology, orthopedics, cardiovascular diseases, and neurology. Oncology holds the largest share within the application segment, driven by the rising incidence of cancers across the Asia Pacific region and the increasing acceptance of cell therapies in cancer treatments such as CAR-T therapies. Ongoing clinical trials and FDA approvals in leading markets, such as Japan and China, are further cementing oncology position as a dominant application segment.
Asia-Pacific Cell Therapy Market Competitive Landscape
The Asia Pacific cell therapy market is dominated by a few key players that have established strong footholds through their research advancements and innovative product portfolios. These companies have actively engaged in strategic partnerships and collaborations to extend their reach across the region. The competitive landscape includes both local biotech firms and global pharmaceutical giants, making it a highly consolidated market.
|
Company |
Year Established |
Headquarters |
No. of Employees |
R&D Investments |
Clinical Trials (Ongoing) |
Partnerships |
Product Pipeline |
Manufacturing Capabilities |
|
Fujifilm Cellular Dynamics |
2014 |
Japan |
- |
- |
- |
- |
- |
- |
|
Novartis AG |
1996 |
Switzerland |
- |
- |
- |
- |
- |
- |
|
Gilead Sciences (Kite Pharma) |
2009 |
USA |
- |
- |
- |
- |
- |
- |
|
Takeda Pharmaceutical |
1781 |
Japan |
- |
- |
- |
- |
- |
- |
|
Mesoblast Ltd. |
2004 |
Australia |
- |
- |
- |
- |
- |
- |
Asia-Pacific Cell Therapy Market Analysis
Asia-Pacific Cell Therapy Market Growth Drivers
- Advancements in Stem Cell Research: The Asia-Pacific region is witnessing rapid advancements in stem cell research, bolstered by strong government support and funding. Countries like Japan and South Korea have established leading research institutions focusing on regenerative medicine. In 2024, Japans Ministry of Health, Labour, and Welfare allocated over USD 350 million to support regenerative medicine research, including stem cells. Additionally, South Korea's government is investing heavily, with a reported USD 500 million earmarked for biotechnology research in 2023. This continued focus on stem cell research aims to accelerate clinical applications in treating chronic diseases.
- Rising Prevalence of Chronic Diseases (Cancer, Cardiovascular): Chronic diseases such as cancer and cardiovascular disorders are on the rise in the Asia-Pacific region, creating a growing demand for cell therapies. According to the World Health Organization, more than 8.2 million people in the region are diagnosed with cancer annually, with a substantial portion requiring advanced treatments like cell therapy. Cardiovascular diseases, the leading cause of death, affect over 13 million people in India alone. This increase in chronic disease prevalence highlights the urgent need for innovative medical solutions like cell-based therapies.
- Increasing Private Investments in Biotech R&D: Private investments in the Asia-Pacific biotech sector, particularly in cell therapy, have surged. In 2024, South Korea witnessed a total private sector investment of USD 1.3 billion into biotech startups, many of which focus on cell and gene therapy. Meanwhile, India's biotech sector attracted over USD 900 million in foreign direct investments in 2023. This influx of capital into biotech R&D is helping accelerate the development of new therapies, making Asia-Pacific a key hub for the global cell therapy market.
Asia-Pacific Cell Therapy Market Challenges
- High Costs of Cell Therapy: The high cost of cell therapy remains a major barrier in the Asia-Pacific region. In 2023, the average cost of a stem cell therapy procedure ranged from USD 25,000 to USD 100,000, making it inaccessible for a large portion of the population. Countries like India and the Philippines, where healthcare affordability is a substantial issue, face challenges in the widespread adoption of these therapies. Governments are working toward subsidizing these costs, but financial barriers continue to hinder market growth.
- Regulatory Complexities Across the Region: The regulatory landscape for cell therapy varies substantially across the Asia-Pacific region, creating challenges for companies looking to operate in multiple countries. In Japan, for instance, regulatory approvals are relatively fast, but in India and China, the process remains slow due to bureaucratic hurdles. In 2023, only 60% of cell therapy products submitted for approval in India received clearance, compared to 80% in Japan. These disparities make it difficult for companies to scale their operations regionally.
Asia-Pacific Cell Therapy Future Market Outlook
Over the next five years, the Asia Pacific cell therapy market is expected to witness substantial growth driven by increasing demand for personalized medicine and advancements in gene-editing technologies such as CRISPR. The support from government initiatives for cell therapy research and manufacturing will further boost market development, particularly in countries like Japan, South Korea, and China. As more therapies receive regulatory approval and enter clinical practice, the market will continue to evolve with innovations in immunotherapies and precision medicine.
Asia-Pacific Cell Therapy Market Opportunities:
- Expansion in Emerging Markets (India, China): Emerging markets in the Asia-Pacific region, particularly India and China, present immense growth opportunities for the cell therapy market. China, with its booming healthcare sector, conducted over 3,000 clinical trials for cell therapies in 2023, while India's National Biopharma Mission has allocated more than USD 1 billion to foster biotech innovations. These initiatives create fertile ground for expanding the cell therapy market, especially as governments push for increased accessibility and affordability of such treatments.
- Potential in Immunotherapy Applications: Cell-based immunotherapies, particularly CAR-T cell treatments, are gaining traction in the Asia-Pacific region. In 2023, over 500 CAR-T therapy trials were conducted across China, Japan, and South Korea, with a focus on treating blood cancers. As immunotherapy emerges as a leading solution for oncology treatments, cell therapy companies in the region are increasingly focusing on expanding their research and development in this domain, offering lucrative market opportunities in the coming years.
Scope of the Report
|
By Therapy Type |
Allogeneic Therapy Autologous Therapy |
|
By Cell Type |
Stem Cells (Mesenchymal Hematopoietic Induced Pluripotent Stem Cells [iPSCs]) Immune Cells (T-Cells, Natural Killer [NK] Cells) |
|
By Application |
Oncology Orthopedics Cardiovascular Diseases Neurology |
|
By End-User |
Hospitals & Clinics Research Institutes Academic & Research Centers |
|
By Region |
China Japan India Australia South Korea Rest of APAC |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Government and Regulatory Bodies
Banks and Financial Institutes
Investors and Venture Capitalists
Biopharmaceutical Companies
Healthcare Companies
Venture Capital Firms
Biotech Manufacturing Firms
Time Period Captured in the Report
Historical Period: 2018-2023
Base Year: 2023
Forecast Period: 2023-2028
Companies
Players Mentioned in the Report:
Fujifilm Cellular Dynamics
Novartis AG
Gilead Sciences (Kite Pharma)
Takeda Pharmaceutical
Mesoblast Ltd.
Vericel Corporation
Bluebird Bio
JCR Pharmaceuticals Co. Ltd.
Thermo Fisher Scientific
Pluristem Therapeutics
Osiris Therapeutics
Athersys, Inc.
Anterogen Co., Ltd.
Chiesi Farmaceutici S.p.A.
Cell Medica
Table of Contents
1. Asia Pacific Cell Therapy Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (Stem Cell Therapy, CAR-T Therapy, Mesenchymal Cell Therapy)
1.4. Market Segmentation Overview
2. Asia Pacific Cell Therapy Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis (Chimeric Antigen Receptor [CAR] T-cell Therapy, Hematopoietic Stem Cell Transplantation [HSCT])
2.3. Key Market Developments and Milestones
3. Asia Pacific Cell Therapy Market Analysis
3.1. Growth Drivers
3.1.1. Advancements in Stem Cell Research
3.1.2. Rising Prevalence of Chronic Diseases (Cancer, Cardiovascular)
3.1.3. Supportive Government Initiatives & Funding
3.1.4. Increasing Private Investments in Biotech R&D
3.2. Market Challenges
3.2.1. High Costs of Cell Therapy
3.2.2. Regulatory Complexities Across the Region
3.2.3. Limited Skilled Workforce and Technical Expertise
3.3. Opportunities
3.3.1. Expansion in Emerging Markets (India, China)
3.3.2. Potential in Immunotherapy Applications
3.3.3. Collaborations Between Academia and Industry
3.4. Trends
3.4.1. Personalized and Precision Cell Therapies
3.4.2. Adoption of Gene-Edited Cell Therapies
3.4.3. Use of AI for Enhancing Cell Therapy Research
3.5. Government Regulation
3.5.1. FDA Guidelines for Cell-Based Therapies
3.5.2. Japans Fast-Track Approval for Regenerative Medicine
3.5.3. National Clinical Trials Networks
3.5.4. Public-Private Partnership for Innovation (Australia, South Korea)
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. Asia Pacific Cell Therapy Market Segmentation
4.1. By Therapy Type (In Value %)
4.1.1. Allogeneic Therapy
4.1.2. Autologous Therapy
4.2. By Cell Type (In Value %)
4.2.1. Stem Cells (Mesenchymal, Hematopoietic, Induced Pluripotent Stem Cells [iPSCs])
4.2.2. Immune Cells (T-Cells, Natural Killer [NK] Cells)
4.3. By Application (In Value %)
4.3.1. Oncology
4.3.2. Orthopedics
4.3.3. Cardiovascular Diseases
4.3.4. Neurology
4.4. By End-User (In Value %)
4.4.1. Hospitals & Clinics
4.4.2. Research Institutes
4.4.3. Academic & Research Centers
4.5. By Region (In Value %)
4.5.1. China
4.5.2. Japan
4.5.3. India
4.5.4. Australia
4.5.5. South Korea
5. Asia Pacific Cell Therapy Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1. Fujifilm Cellular Dynamics
5.1.2. Novartis AG
5.1.3. Gilead Sciences (Kite Pharma)
5.1.4. Thermo Fisher Scientific
5.1.5. Bluebird Bio
5.1.6. Cell Medica
5.1.7. JCR Pharmaceuticals Co. Ltd.
5.1.8. Vericel Corporation
5.1.9. Osiris Therapeutics
5.1.10. Pluristem Therapeutics
5.1.11. Takeda Pharmaceutical Company
5.1.12. Anterogen Co., Ltd.
5.1.13. Chiesi Farmaceutici S.p.A.
5.1.14. Mesoblast Ltd.
5.1.15. Athersys, Inc.
5.2 Cross Comparison Parameters (Market Presence, Clinical Trials, Regulatory Approvals, Manufacturing Capabilities, R&D Investments, Partnerships, Innovation Focus, Revenue Growth)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers And Acquisitions
5.6 Investment Analysis
5.7 Venture Capital Funding
5.8 Government Grants
5.9 Private Equity Investments
6. Asia Pacific Cell Therapy Market Regulatory Framework
6.1. Regulatory Agencies Overview (Japan Pharmaceuticals and Medical Devices Agency [PMDA], India CDSCO, China NMPA)
6.2. Compliance Requirements for Cell-Based Therapies
6.3. Certification and Licensing Procedures
7. Asia Pacific Cell Therapy Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. Asia Pacific Cell Therapy Future Market Segmentation
8.1. By Therapy Type (In Value %)
8.2. By Cell Type (In Value %)
8.3. By Application (In Value %)
8.4. By End-User (In Value %)
8.5. By Region (In Value %)
9. Asia Pacific Cell Therapy Market Analysts Recommendations
9.1. Total Addressable Market (TAM), Serviceable Available Market (SAM), Serviceable Obtainable Market (SOM) Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives for Stakeholders
9.4. White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The initial step involves the identification of critical market drivers, such as the adoption of regenerative medicine, government policies, and patient demographics. This information is gathered through both primary and secondary research channels, including government reports and healthcare industry databases.
Step 2: Market Analysis and Construction
In this phase, the market is analyzed through a combination of historical data and current trends in the cell therapy market. This includes understanding the volume of clinical trials, therapy adoption rates, and the types of therapies being commercialized across different countries in the Asia Pacific region.
Step 3: Hypothesis Validation and Expert Consultation
Industry experts from key companies are consulted to validate the research hypotheses. This step provides insights into emerging trends, innovations in therapy development, and key challenges companies face in bringing cell therapies to market.
Step 4: Research Synthesis and Final Output
The final research output includes compiling all validated data from industry consultations, historical data, and financial reports. This step ensures a comprehensive and accurate representation of the Asia Pacific cell therapy market.
Frequently Asked Questions
01. How big is the Asia Pacific Cell Therapy Market?
The Asia Pacific cell therapy market is valued at USD 1.5 billion, driven by the rising demand for innovative treatment options for chronic diseases like cancer and cardiovascular conditions. The market benefits from strong government support and private investments in cell therapy research.
02. What are the challenges in the Asia Pacific Cell Therapy Market?
The Asia Pacific cell therapy market challenges include high treatment costs, regulatory hurdles, and the complexity of cell therapy manufacturing. Additionally, the lack of skilled professionals and the time-intensive process for therapy development are substantial obstacles in the market.
03. Who are the major players in the Asia Pacific Cell Therapy Market?
The Asia Pacific cell therapy market key players include Fujifilm Cellular Dynamics, Novartis AG, Gilead Sciences (Kite Pharma), Takeda Pharmaceutical, and Mesoblast Ltd. These companies have established strong footholds due to their innovative product pipelines and strategic collaborations.
04. What are the growth drivers of the Asia Pacific Cell Therapy Market?
The Asia Pacific cell therapy market growth is driven by advancements in stem cell research, increasing demand for personalized medicine, and government initiatives supporting the development of regenerative medicine. The adoption of cutting-edge technologies, such as gene editing, also plays a crucial role in market expansion.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















