
Asia Pacific Clinical Trials Market Outlook to 2030
Region:Asia
Author(s):Meenakshi Bisht
Product Code:KROD9868
December 2024
88
About the Report
Asia Pacific Clinical Trials Market Overview
- The Asia Pacific Clinical Trials Market is valued at USD 15.5 billion, driven by a growing demand for efficient drug development, particularly in the fields of oncology, cardiology, and infectious diseases. The regions extensive patient population, coupled with increased healthcare expenditure, has encouraged pharmaceutical and biotechnology companies to conduct clinical trials in this region.
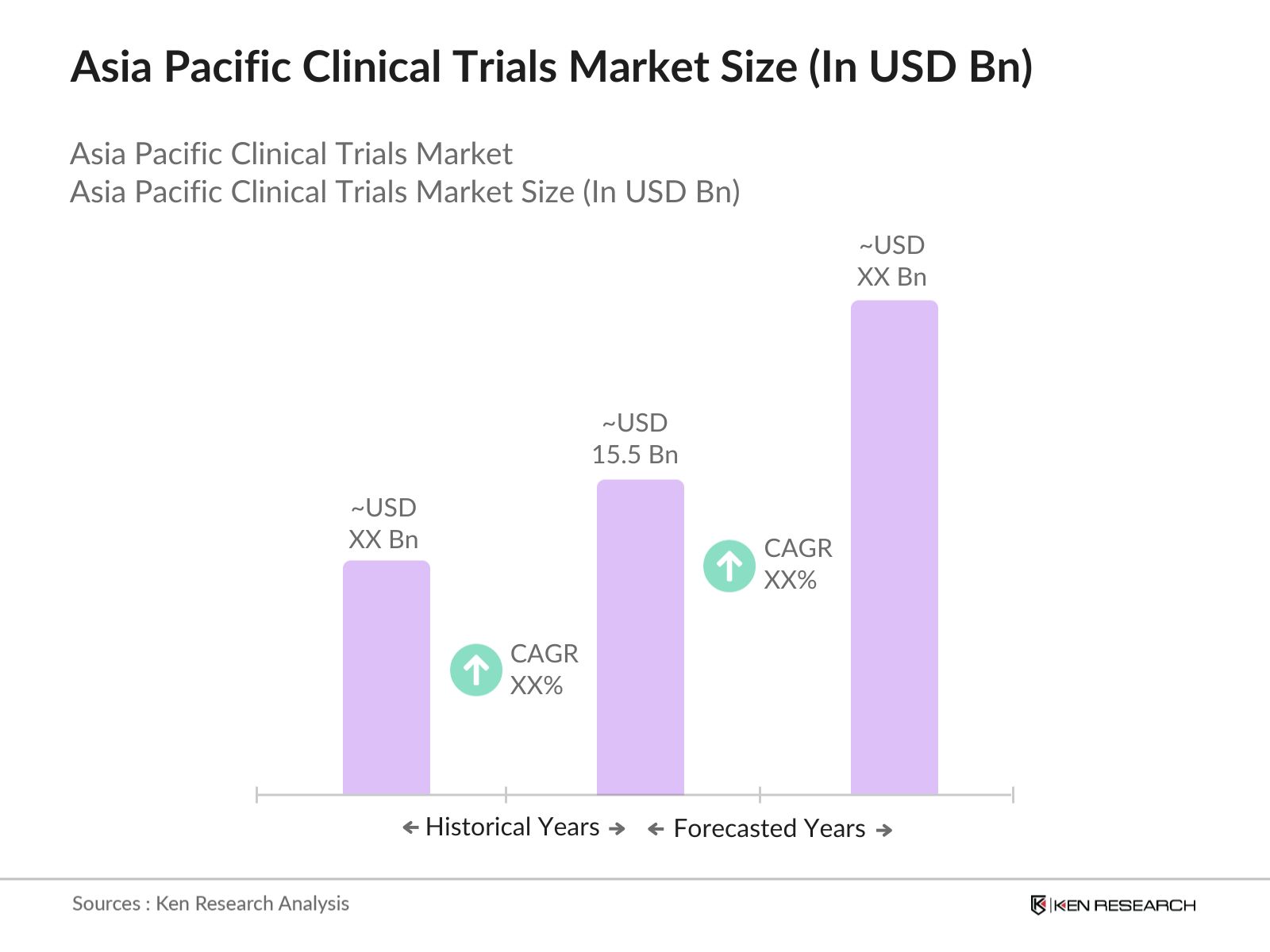
- The market is dominated by countries like China, India, and Japan. China and India are favored due to their large populations, which provides a vast patient pool for clinical trials. Both nations also benefit from government support in research and development, along with lower operational costs. Japan leads in clinical research, particularly in high-end therapeutic areas like oncology and neurology, due to its advanced healthcare infrastructure and well-established regulatory framework, making it a preferred hub for clinical trials in the region.
- Fast-track approvals and orphan drug designations have accelerated the clinical trial process in the Asia Pacific region. By 2023, Japan had approved over 50 orphan drugs using fast-track processes, while China had processed more than 40 rare disease drugs through similar programs. These designations help reduce the time and cost of bringing life-saving therapies to market for rare conditions, enhancing patient outcomes.
Asia Pacific Clinical Trials Market Segmentation
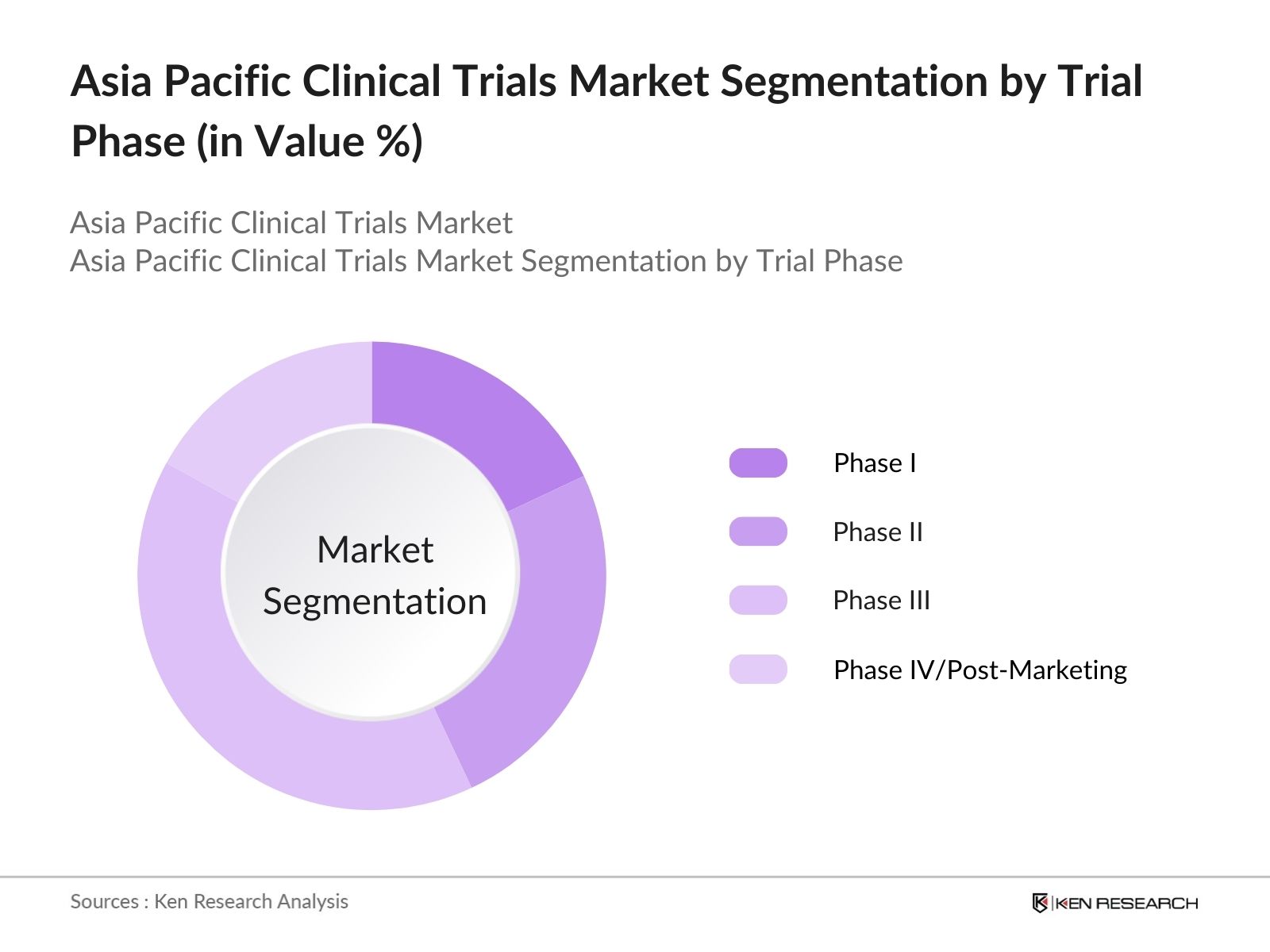
By Trial Phase: The Asia Pacific clinical trials market is segmented by trial phase into Phase I, Phase II, Phase III, and Phase IV/Post-Marketing Surveillance. The Phase III trials dominate the market due to the critical role they play in evaluating the efficacy and safety of new drugs before they reach regulatory approval. These trials involve large-scale patient participation, which increases the reliability of the data generated. The large patient pool available in countries like India and China, along with their cost-effective operations, has positioned these nations as leading destinations for Phase III trials. This phase requires a significant investment of resources and time, further solidifying its dominance in the market.
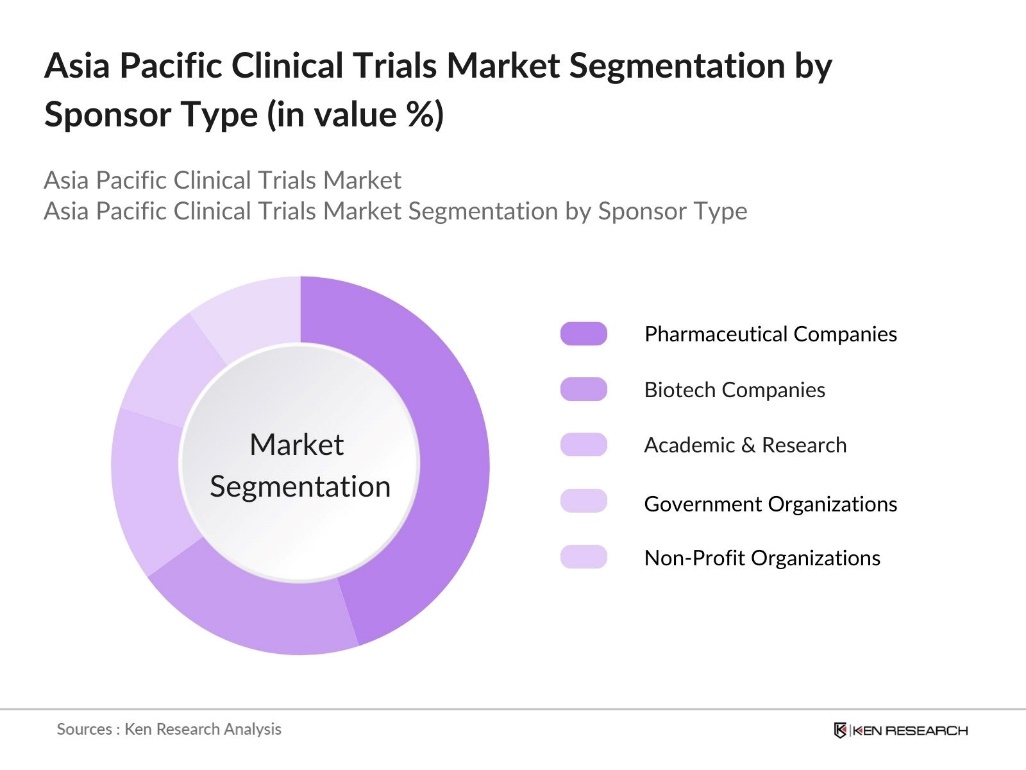
By Sponsor Type: The Asia Pacific clinical trials market is segmented by sponsor type into pharmaceutical companies, biotech companies, academic and research institutions, government organizations, and non-profit organizations. The Pharmaceutical companies have the dominant market share in this segment due to their heavy involvement in drug development and commercialization. Large pharmaceutical firms are key sponsors of clinical trials, often outsourcing them to Contract Research Organizations (CROs) to reduce costs and expedite the process. Their substantial financial resources, coupled with the need for continuous innovation, drive the dominance of this sub-segment in the market.
Asia Pacific Clinical Trials Market Competitive Landscape
The consolidation of key players in this market highlights the strong presence of established CROs and pharmaceutical giants. Companies such as IQVIA and Syneos Health dominate due to their global networks and comprehensive service offerings, ranging from early-phase clinical trials to post-market surveillance. Furthermore, regional players like Novotech and George Clinical leverage their understanding of local regulatory environments to offer specialized services tailored to the needs of Asia Pacific.
|
Company |
Establishment Year |
Headquarters |
CRO Capabilities |
Therapeutic Focus |
No. of Clinical Sites |
Revenue (USD) |
Key Markets |
Technology Use |
|
IQVIA |
1982 |
Durham, USA |
||||||
|
PPD (Thermo Fisher) |
1985 |
Wilmington, USA |
||||||
|
Syneos Health |
1998 |
Morrisville, USA |
||||||
|
Novotech |
1996 |
Sydney, Australia |
||||||
|
George Clinical |
2005 |
Sydney, Australia |
Asia Pacific Clinical Trials Industry Analysis
Growth Drivers
- Increasing Prevalence of Chronic Diseases: The Asia Pacific region has seen a significant rise in chronic diseases. In China, the incidence of cancer reached 4.82 million in 2022, contributing heavily to the demand for clinical trials in the region. This demand for more trial-based interventions has driven research, particularly in oncology and cardiovascular diseases. The increased prevalence is pushing the need for innovative therapeutic solutions through clinical research to address these urgent health challenges.
- Adoption of Decentralized Clinical Trials (DCTs): The adoption of decentralized clinical trials (DCTs) is accelerating due to technological advancements in the Asia Pacific region. India and Japan have been at the forefront of implementing DCT models in 2023 leveraging decentralized platforms to reduce costs and improve patient access. The growth of internet penetration in rural areas of China and India has enabled broader participation in clinical trials, making the Asia Pacific region a leader in decentralized clinical models.
- Rising Demand for Personalized Medicine: The growing demand for personalized medicine is reshaping clinical trials in the Asia Pacific region. With a shift toward targeted therapies and precision treatments, trials increasingly focus on genomics and individualized patient care. This trend is transforming how trials are designed and conducted, emphasizing tailored solutions that cater to specific patient needs, thereby influencing regulatory frameworks and trial methodologies in the region.
Market Challenges
- Regulatory Variability Across Countries: Regulatory inconsistencies across Asia Pacific countries present significant challenges for clinical trials. While some nations have streamlined approval processes, others face slower and more complex regulatory frameworks. This variability in ethics committee standards and regulatory approvals can result in delays and higher costs for multinational trials. Such inconsistencies make it difficult for global pharmaceutical companies to efficiently conduct trials across multiple countries in the region.
- High Operational Costs in Clinical Trials: Clinical trials in the Asia Pacific region experience high operational costs, particularly in developed markets. Expenses related to site management and monitoring are significant, especially in countries with advanced healthcare systems. While emerging markets offer more cost-effective options, they often face infrastructure challenges. These cost disparities influence the scalability of trials, complicating their execution across diverse regions.
Asia Pacific Clinical Trials Market Future Outlook
Over the next five years, the Asia Pacific clinical trials market is expected to experience robust growth, driven by the rising prevalence of chronic diseases, advancements in clinical trial technology, and a growing patient population. Increasing investments from both government and private sectors into drug research and development, especially in emerging markets like India and Southeast Asia, will further accelerate the markets expansion. Furthermore, the adoption of decentralized clinical trials (DCTs), along with the integration of real-world evidence (RWE) and AI, is anticipated to revolutionize the way clinical trials are conducted in the region.
Market Opportunities
- Growth of CROs (Contract Research Organizations): The Asia Pacific region is experiencing substantial growth in Contract Research Organizations (CROs), playing a crucial role in the expansion of clinical trials. CROs help pharmaceutical companies streamline trial operations by offering outsourcing solutions for trial management. The region's favorable conditions, including lower operational costs and access to a large patient population, make it an attractive market for CROs to scale their operations and support clinical research growth.
- Expansion into Emerging Markets: Emerging markets in the Asia Pacific, such as India, China, and Southeast Asia, offer significant potential for clinical trials. These regions present large patient populations and improving healthcare infrastructures, creating opportunities for multinational pharmaceutical companies to conduct trials. As healthcare systems in these countries continue to evolve, they remain attractive destinations for clinical research investments, supporting the expansion of global trials in the region.
Scope of the Report
|
By Trial Phase |
Phase I Phase II Phase III Phase IV/Post-Marketing Surveillance |
|
By Sponsor Type |
Pharmaceutical Companies Biotech Companies, Academic and Research Institutions Government Organizations Non-Profit Organizations |
|
By Therapeutic Indication |
Oncology Cardiovascular Diseases Neurological Disorders Infectious Diseases Rare Diseases |
|
By Technology |
Decentralized Clinical Trials Wearable Devices in Trials Electronic Data Capture Systems AI-Powered Trial Design |
|
By Region |
China India Japan South Korea Australia |
Products
Key Target Audience
Pharmaceutical Companies
Biotechnology Firms
Contract Research Organizations (CROs)
Medical Device Manufacturers
Government and Regulatory Bodies (e.g., PMDA, CDSCO, TGA)
Investors and venture capital Firms
Banks and Financial Institutions
Companies
Players Mentioned in the Report
IQVIA
PPD (Thermo Fisher)
Syneos Health
LabCorp Drug Development
Parexel
ICON plc
Medpace
Wuxi AppTec
Charles River Laboratories
Covance
Table of Contents
1. Asia Pacific Clinical Trials Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy (Clinical Trials Phases, Sponsors, Indications, and Geographies)
1.3 Market Growth Rate
1.4 Market Segmentation Overview
2. Asia Pacific Clinical Trials Market Size (In USD Bn)
2.1 Historical Market Size
2.2 Year-On-Year Growth Analysis
2.3 Key Market Developments and Milestones
3. Asia Pacific Clinical Trials Market Analysis
3.1 Growth Drivers
3.1.1 Increasing Prevalence of Chronic Diseases
3.1.2 Government Initiatives Supporting Research
3.1.3 Adoption of Decentralized Clinical Trials (DCTs)
3.1.4 Rising Demand for Personalized Medicine
3.2 Market Challenges
3.2.1 Regulatory Variability Across Countries (Ethics Committees, Regulatory Approvals)
3.2.2 High Operational Costs in Clinical Trials (Per Patient Cost, Site Management Costs)
3.2.3 Limited Access to Patient Populations
3.2.4 Delayed Trial Timelines Due to Patient Recruitment Issues
3.3 Opportunities
3.3.1 Growth of CROs (Contract Research Organizations)
3.3.2 Expansion into Emerging Markets (India, China, Southeast Asia)
3.3.3 Technological Advancements (Wearables, Remote Monitoring, AI in Trial Designs)
3.3.4 Increasing Collaboration between Pharma Companies and Academic Institutions
3.4 Trends
3.4.1 Adoption of Real-World Evidence (RWE) in Trial Design
3.4.2 Shift Towards Adaptive Trial Designs
3.4.3 Integration of Digital Health in Patient Monitoring
3.4.4 Rise of Virtual/Hybrid Clinical Trials (Remote Recruitment, Telemedicine)
3.5 Government Regulation
3.5.1 Regulatory Harmonization Initiatives in Asia Pacific (ICH-GCP Adoption)
3.5.2 Fast Track and Orphan Drug Designations (Impact on Approval Process)
3.5.3 Cross-Border Clinical Trials Regulations (APEC, ASEAN)
3.5.4 Public-Private Partnerships in Research
3.6 SWOT Analysis
3.7 Stakeholder Ecosystem
3.8 Porters Five Forces
3.9 Competitive Ecosystem
4. Asia Pacific Clinical Trials Market Segmentation
4.1 By Trial Phase (In Value %)
4.1.1 Phase I
4.1.2 Phase II
4.1.3 Phase III
4.1.4 Phase IV/Post-Marketing Surveillance
4.2 By Sponsor Type (In Value %)
4.2.1 Pharmaceutical Companies
4.2.2 Biotech Companies
4.2.3 Academic and Research Institutions
4.2.4 Government Organizations
4.2.5 Non-Profit Organizations
4.3 By Therapeutic Indication (In Value %)
4.3.1 Oncology
4.3.2 Cardiovascular Diseases
4.3.3 Neurological Disorders
4.3.4 Infectious Diseases
4.3.5 Rare Diseases
4.4 By Technology (In Value %
4.4.1 Decentralized Clinical Trials
4.4.2 Wearable Devices in Trials
4.4.3 Electronic Data Capture Systems
4.4.4 AI-Powered Trial Design
4.5 By Geography (In Value %)
4.5.1 China
4.5.2 India
4.5.3 Japan
4.5.4 South Korea
4.5.5 Australia
5. Asia Pacific Clinical Trials Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1 IQVIA
5.1.2 PPD (Thermo Fisher)
5.1.3 LabCorp Drug Development
5.1.4 Parexel
5.1.5 Syneos Health
5.1.6 Covance
5.1.7 ICON plc
5.1.8 PRA Health Sciences
5.1.9 Medpace
5.1.10 Wuxi AppTec
5.1.11 Charles River Laboratories
5.1.12 KCR
5.1.13 Clinipace
5.1.14 Novotech
5.1.15 George Clinical
5.2 Cross Comparison Parameters (No. of Employees, Headquarters, Inception Year, Revenue, Clinical Trial Expertise, Therapeutic Focus, Key Markets, CRO vs In-House Model)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers and Acquisitions
5.6 Investment Analysis
5.7 Venture Capital Funding
5.8 Government Grants
5.9 Private Equity Investments
6. Asia Pacific Clinical Trials Market Regulatory Framework
6.1 Regulatory Compliance in APAC Region
6.2 Certification Processes for Clinical Trials
6.3 Ethical Considerations and Patient Consent Laws
6.4 Key Regulatory Authorities (PMDA, CDSCO, TGA, etc.)
7. Asia Pacific Clinical Trials Future Market Size (In USD Bn)
7.1 Future Market Size Projections
7.2 Key Factors Driving Future Market Growth
8. Asia Pacific Clinical Trials Future Market Segmentation
8.1 By Trial Phase (In Value %)
8.2 By Sponsor Type (In Value %)
8.3 By Therapeutic Indication (In Value %)
8.4 By Technology (In Value %)
8.5 By Geography (In Value %)
9. Asia Pacific Clinical Trials Market Analysts Recommendations
9.1 TAM/SAM/SOM Analysis
9.2 Customer Cohort Analysis
9.3 Market Entry Strategies for APAC
9.4 White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The initial phase involved identifying major stakeholders in the Asia Pacific clinical trials market. A combination of secondary research using databases such as World Bank, WHO, and proprietary resources helped pinpoint critical factors like patient population, therapeutic demand, and regulatory trends.
Step 2: Market Analysis and Construction
Historical data pertaining to clinical trial activity in Asia Pacific was gathered and analyzed to understand key market dynamics. Data sources included trial registries, revenue reports, and regional healthcare expenditure.
Step 3: Hypothesis Validation and Expert Consultation
The market data was further validated through expert consultations with representatives from CROs, pharmaceutical firms, and healthcare providers. Insights from these consultations helped refine the estimates and understand emerging trends like DCTs.
Step 4: Research Synthesis and Final Output
Finally, all gathered data was synthesized and cross-verified using bottom-up and top-down approaches, ensuring an accurate representation of the Asia Pacific clinical trials market.
Frequently Asked Questions
01 How big is the Asia Pacific Clinical Trials Market?
The Asia Pacific Clinical Trials Market is valued at USD 15.5 billion, driven by the increasing need for drug development and research activities, particularly in areas like oncology and cardiology.
02 What are the challenges in the Asia Pacific Clinical Trials Market?
Challenges in Asia Pacific clinical trials market include regulatory complexities across countries, high operational costs, and issues related to patient recruitment. Furthermore, limited access to advanced infrastructure in some countries also hampers trial efficiency.
03 Who are the major players in the Asia Pacific Clinical Trials Market?
Key players in the Asia Pacific clinical trials market include IQVIA, PPD (Thermo Fisher), Syneos Health, Novotech, and George Clinical. These companies lead due to their comprehensive service offerings and deep regional expertise.
04 What are the growth drivers of the Asia Pacific Clinical Trials Market?
The Asia Pacific Clinical Trials Market is driven by the growing prevalence of chronic diseases, government initiatives to promote R&D, and the increasing adoption of decentralized clinical trials.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















