
Asia Pacific Point Of Care Diagnostics Market Outlook to 2030
Region:Asia
Author(s):Naman Rohilla
Product Code:KROD7459
December 2024
85
About the Report
Asia Pacific Point of Care Diagnostics Market Overview
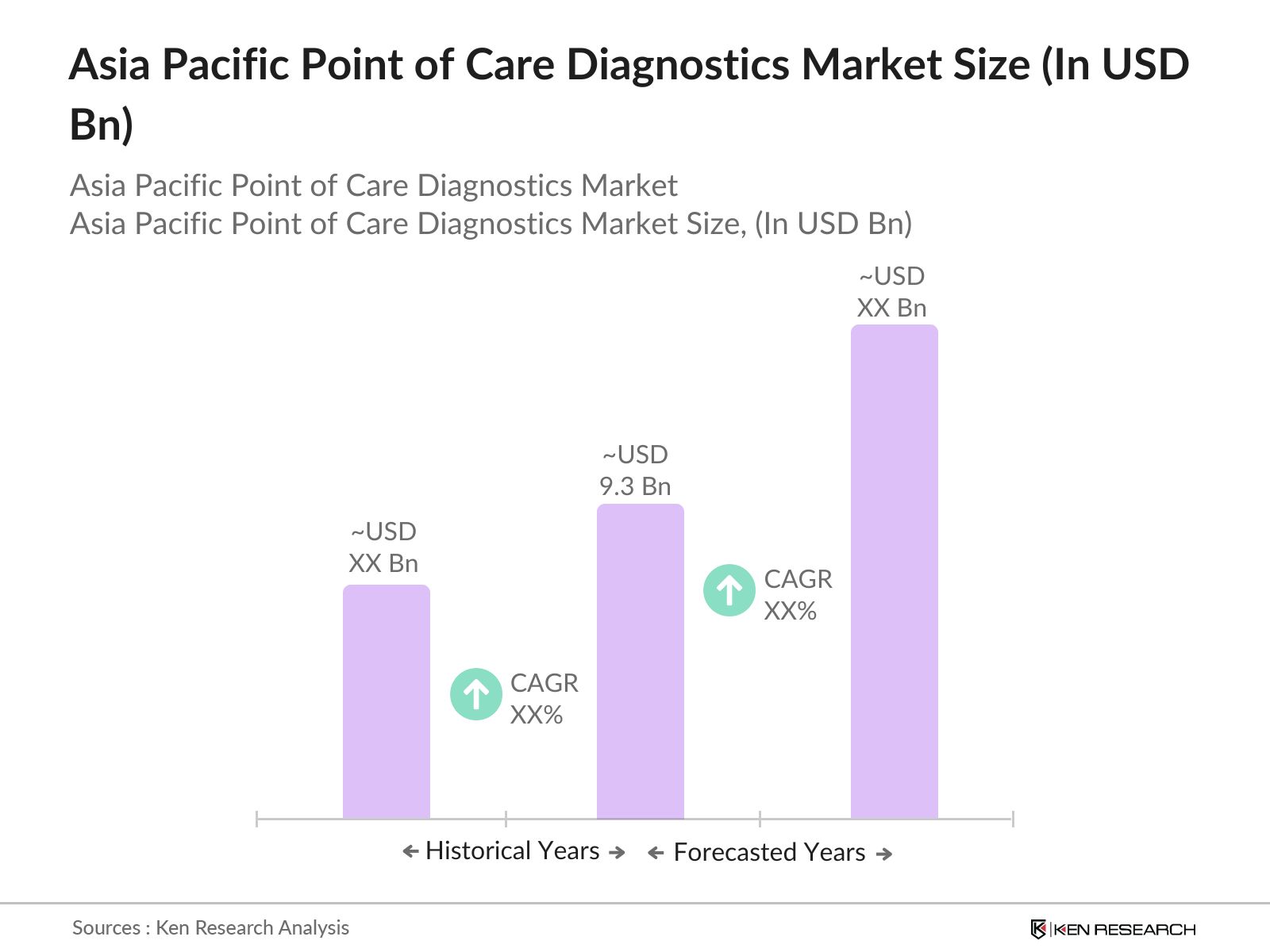
- The Asia Pacific Point of Care Diagnostics (POC) market is valued at USD 9.3 billion based on a five-year historical analysis. This growth is driven by the increasing demand for rapid diagnostic testing, fueled by the rising prevalence of chronic diseases such as diabetes, cardiovascular disorders, and infectious diseases. A growing focus on early diagnosis and improved patient outcomes through minimally invasive diagnostic procedures also drives market expansion. The need for decentralized testing capabilities in remote areas further adds to the market's acceleration.
- Countries like China, India, and Japan dominate the Asia Pacific Point of Care Diagnostics market due to their high population density, expanding healthcare infrastructure, and increased government support for improving healthcare accessibility. China leads the market with substantial investments in healthcare innovations and initiatives to improve public health. Similarly, Japan's aging population has resulted in a greater demand for diagnostic tools, while India's rapidly growing healthcare sector fosters growth due to higher disease incidence and increasing healthcare awareness.
- The Asia Pacific medical device regulatory framework is complex, with varying requirements across countries. In 2023, only 11 out of 48 Asia Pacific countries had fully implemented the Asia Pacific Medical Device Harmonization Initiative (APMDHI) guidelines. This inconsistency in regulatory standards creates barriers to entry for POC diagnostic manufacturers, as they must meet different certification and approval requirements in each country. However, ongoing efforts to harmonize regulations across the region aim to streamline the approval process and accelerate market access for diagnostic devices.
Asia Pacific Point of Care Diagnostics Market Segmentation
- By Product Type: The Asia Pacific Point of Care Diagnostics market is segmented by product type into glucose monitoring kits, infectious disease testing kits, cardiac markers, pregnancy and fertility testing kits, and blood gas/electrolytes analysis devices. Among these, glucose monitoring kits dominate the market, attributed to the growing prevalence of diabetes in countries like India and China. With the increasing awareness of diabetes management and the adoption of self-monitoring tools, glucose monitoring kits have become widely accepted among healthcare providers and patients. The demand for these kits is further bolstered by technological advancements and the convenience of real-time blood glucose monitoring.

- By Technology: The market is also segmented by technology into lateral flow assays, polymerase chain reaction (PCR), microfluidics, immunoassays, and biosensors. Immunoassays hold the largest market share within this segment, driven by their application in various diagnostic tests, including infectious diseases, oncology, and cardiology. The simplicity, cost-effectiveness, and rapid turnaround time of immunoassays make them a preferred choice for point-of-care diagnostics in resource-limited settings, especially in countries like India and Southeast Asia, where access to centralized laboratories is limited.

Asia Pacific Point of Care Diagnostics Market Competitive Landscape
The Asia Pacific Point of Care Diagnostics market is dominated by several key players that have established a strong foothold due to their product innovations, extensive distribution networks, and strategic partnerships. Companies like Roche Diagnostics, Abbott Laboratories, and Siemens Healthineers lead the market with a robust portfolio of diagnostic products and services.
|
Company |
Year Established |
Headquarters |
Product Portfolio |
Regional Presence |
R&D Spending |
Technology Integration |
Strategic Partnerships |
Revenue |
|
Roche Diagnostics |
1896 |
Basel, Switzerland |
- |
- |
- |
- |
- |
- |
|
Abbott Laboratories |
1888 |
Illinois, USA |
- |
- |
- |
- |
- |
- |
|
Siemens Healthineers |
1847 |
Erlangen, Germany |
- |
- |
- |
- |
- |
- |
|
Danaher Corporation |
1984 |
Washington, USA |
- |
- |
- |
- |
- |
- |
|
Becton, Dickinson and Company |
1897 |
New Jersey, USA |
- |
- |
- |
- |
- |
- |
Asia Pacific Point of Care Diagnostics Market Analysis
Asia Pacific Point of Care Diagnostics Market Growth Drivers
- Growing Prevalence of Chronic Diseases: The rising prevalence of chronic diseases such as diabetes, cardiovascular diseases, and cancer is a growth driver for the Asia Pacific point-of-care diagnostics (POC) market. In 2023, the International Diabetes Federation reported that 206 million adults in the region have diabetes, demanding more efficient diagnostics. Additionally, the World Health Organization (WHO) noted that cardiovascular diseases account for 9.4 million deaths annually in the region. The increasing burden of these conditions accelerates the adoption of POC diagnostics for early and rapid diagnosis. These technologies enable faster detection and better management of chronic diseases in hospital and outpatient settings.
- Increasing Demand for Rapid Diagnostics: The demand for rapid diagnostics has surged as healthcare systems strive to provide quicker turnaround times for disease detection, particularly in regions with limited infrastructure. The WHO estimated that 70% of the rural population in Asia Pacific lacks access to timely laboratory testing, pushing healthcare providers toward POC diagnostics, which can deliver results in under 30 minutes. This has led to a higher uptake of rapid diagnostic technologies, especially in emergency rooms and clinics in rural areas. The availability of faster diagnostics reduces hospital stays and improves patient management across the region.
- Technological Advancements in Diagnostics: Technological advancements are revolutionizing the POC diagnostics market in Asia Pacific. The introduction of portable devices and integration with mobile health solutions has accelerated diagnostics at remote and mobile health centers. By 2024, over 1.5 billion mobile users across Asia Pacific are expected to benefit from mobile-enabled diagnostic services, as per data from GSMA. Advancements like biosensors and microfluidics have further improved the accuracy of POC devices, reducing the reliance on traditional diagnostic methods and increasing patient accessibility to these technologies.
Asia Pacific Point of Care Diagnostics Market Challenges
- Regulatory Compliance: Regulatory compliance remains a major challenge for POC diagnostics companies operating in Asia Pacific. Each country in the region has its regulatory frameworks for medical devices, making it difficult for manufacturers to navigate. The Asia Pacific Medical Device Harmonization Initiative (APMDHI) noted that only 38% of countries in the region had harmonized regulatory practices by 2023. This inconsistency creates delays in product approvals, impacting the timely introduction of new POC diagnostic tools into the market. Navigating different certification requirements also increases the cost and time required for market entry.
- Cost Constraints in Emerging Markets: Cost constraints, particularly in emerging markets, pose challenges to the adoption of POC diagnostics. Despite technological advancements, the affordability of devices remains a concern for low-income countries in the region. According to the World Bank, 24% of the Asia Pacific population lives on less than USD 3.20 per day. This economic disparity limits access to POC diagnostic tools, which are often priced higher than traditional methods, particularly in rural areas. Moreover, funding for healthcare infrastructure is often inadequate, further hampering the market's growth in these regions.
Asia Pacific Point of Care Diagnostics Market Future Outlook
Over the next five years, the Asia Pacific Point of Care Diagnostics market is expected to witness growth, driven by increasing investments in healthcare infrastructure, the growing adoption of advanced diagnostic technologies, and rising demand for decentralized healthcare services. The rising burden of chronic diseases such as diabetes and cardiovascular conditions will further fuel demand for point-of-care testing solutions, especially in rural and underdeveloped areas where access to centralized laboratories is limited.
Asia Pacific Point of Care Diagnostics Market Opportunities
- Expansion in Rural Healthcare Infrastructure: The expansion of rural healthcare infrastructure across Asia Pacific presents opportunities for the POC diagnostics market. Governments are increasing investments to improve healthcare access in remote areas. For example, India allocated over USD 2.1 billion in 2023 for rural healthcare development, with a focus on diagnostic services. Similarly, Indonesia announced plans to establish 1,500 new rural healthcare centers by 2025. The deployment of POC diagnostic tools in these new facilities will enhance disease detection and management, particularly for conditions like malaria and tuberculosis, which are prevalent in rural regions.
- Growing Focus on Preventive Healthcare: Preventive healthcare is gaining traction in the Asia Pacific region, leading to increased demand for POC diagnostics. The WHO reported that over 9 million deaths could be prevented annually through early diagnosis and intervention, driving governments to focus on screening and preventive measures. Countries like Japan and South Korea have launched national programs aimed at increasing early diagnosis rates, utilizing POC diagnostics for diseases such as cancer and diabetes. The growing emphasis on preventive care across the region is creating new growth opportunities for POC diagnostic providers.
Scope of the Report
|
By Product Type |
Glucose Monitoring Kits Infectious Disease Testing Kits Cardiac Markers Pregnancy and Fertility Testing Kits Blood Gas/Electrolytes Analysis Devices |
|
By Application |
Hospitals Clinics Homecare Settings Diagnostic Centres Research Laboratories |
|
By Technology |
Lateral Flow Assays Polymerase Chain Reaction (PCR) Microfluidics Immunoassays Biosensors |
|
By End-User |
Public Healthcare Facilities Private Healthcare Providers Academic & Research Institutes Corporate Health Programs NGOs and Government Initiatives |
|
By Region |
China Japan India Australia Southeast Asia |
Products
Key Target Audience
Healthcare Providers
Diagnostic Laboratories
Government and Regulatory Bodies (e.g., National Medical Device Regulatory Authorities, WHO Collaborating Centers)
Hospitals and Clinics
Medical Device Distributors
Banks and Financial Institutions
Investor and Venture Capitalist Firms
Public Healthcare Agencies (e.g., Ministries of Health in APAC countries)
Telemedicine Providers
Companies
Players Mentioned in the Report
Abbott Laboratories
Roche Diagnostics
Siemens Healthineers
Danaher Corporation
Becton, Dickinson and Company
Thermo Fisher Scientific
Johnson & Johnson
PerkinElmer Inc.
QuidelOrtho Corporation
bioMrieux
Table of Contents
1. Asia Pacific Point Of Care Diagnostics Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. Asia Pacific Point Of Care Diagnostics Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. Asia Pacific Point Of Care Diagnostics Market Analysis
3.1. Growth Drivers
3.1.1. Growing Prevalence of Chronic Diseases
3.1.2. Increasing Demand for Rapid Diagnostics
3.1.3. Technological Advancements in Diagnostics
3.1.4. Rising Healthcare Expenditure
3.2. Market Challenges
3.2.1. Regulatory Compliance
3.2.2. Cost Constraints in Emerging Markets
3.2.3. Low Awareness in Remote Areas
3.3. Opportunities
3.3.1. Expansion in Rural Healthcare Infrastructure
3.3.2. Growing Focus on Preventive Healthcare
3.3.3. Adoption of AI and Machine Learning in Diagnostics
3.4. Trends
3.4.1. Integration with Telemedicine
3.4.2. Adoption of Wearable Diagnostic Devices
3.4.3. Increased Use of Multiplex Testing Platforms
3.5. Regulatory Landscape
3.5.1. Asia Pacific Medical Device Regulatory Framework
3.5.2. Point of Care Diagnostics Certification Programs
3.5.3. Compliance with ISO Standards
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem
3.8. Porters Five Forces Analysis
3.9. Competition Ecosystem
4. Asia Pacific Point Of Care Diagnostics Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Glucose Monitoring Kits
4.1.2. Infectious Disease Testing Kits
4.1.3. Cardiac Markers
4.1.4. Pregnancy and Fertility Testing Kits
4.1.5. Blood Gas/Electrolytes Analysis Devices
4.2. By Application (In Value %)
4.2.1. Hospitals
4.2.2. Clinics
4.2.3. Homecare Settings
4.2.4. Diagnostic Centers
4.2.5. Research Laboratories
4.3. By Technology (In Value %)
4.3.1. Lateral Flow Assays
4.3.2. Polymerase Chain Reaction (PCR)
4.3.3. Microfluidics
4.3.4. Immunoassays
4.3.5. Biosensors
4.4. By End-User (In Value %)
4.4.1. Public Healthcare Facilities
4.4.2. Private Healthcare Providers
4.4.3. Academic & Research Institutes
4.4.4. Corporate Health Programs
4.4.5. NGOs and Government Initiatives
4.5. By Region (In Value %)
4.5.1. China
4.5.2. Japan
4.5.3. India
4.5.4. Australia
4.5.5. Southeast Asia
5. Asia Pacific Point Of Care Diagnostics Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Abbott Laboratories
5.1.2. Roche Diagnostics
5.1.3. Siemens Healthineers
5.1.4. Danaher Corporation
5.1.5. Becton, Dickinson and Company
5.1.6. Thermo Fisher Scientific
5.1.7. Johnson & Johnson
5.1.8. PerkinElmer Inc.
5.1.9. QuidelOrtho Corporation
5.1.10. bioMrieux
5.1.11. Hologic Inc.
5.1.12. Sysmex Corporation
5.1.13. Cepheid
5.1.14. Nova Biomedical
5.1.15. EKF Diagnostics
5.2. Cross Comparison Parameters (Revenue, Product Portfolio, Regional Presence, R&D Spending, Technology Integration, Growth Strategy, Customer Base, Strategic Partnerships)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. Asia Pacific Point Of Care Diagnostics Market Regulatory Framework
6.1. Medical Device Regulations
6.2. Import/Export Requirements
6.3. Certification Processes
6.4. Quality and Safety Standards
7. Asia Pacific Point Of Care Diagnostics Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. Asia Pacific Point Of Care Diagnostics Future Market Segmentation
8.1. By Product Type (In Value %)
8.2. By Application (In Value %)
8.3. By Technology (In Value %)
8.4. By End-User (In Value %)
8.5. By Region (In Value %)
9. Asia Pacific Point Of Care Diagnostics Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves identifying key market variables, including diagnostic technologies, product segments, and major stakeholders across the Asia Pacific Point of Care Diagnostics market. This process uses extensive secondary research and proprietary databases to capture relevant industry data and market dynamics.
Step 2: Market Analysis and Construction
In this phase, historical market data is analyzed, focusing on product penetration, the ratio of diagnostic tests performed at point-of-care versus centralized laboratories, and revenue generation across major markets. This includes evaluating both public and private sector participation.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are validated through consultations with industry experts, including senior executives from diagnostic companies, healthcare providers, and regulatory agencies. These insights are obtained through CATI interviews to ensure the robustness of the market estimates and trends.
Step 4: Research Synthesis and Final Output
The final stage involves synthesizing research data from multiple sources, including interviews with point-of-care diagnostic manufacturers, to verify findings and produce a comprehensive report. This process ensures that the analysis is complete and market predictions are accurate.
Frequently Asked Questions
01. How big is the Asia Pacific Point of Care Diagnostics Market?
The Asia Pacific Point of Care Diagnostics market is valued at USD 9.3 billion, driven by increasing demand for rapid diagnostic testing, particularly for chronic diseases such as diabetes and cardiovascular conditions.
02. What are the challenges in the Asia Pacific Point of Care Diagnostics Market?
Challenges in the Asia Pacific Point of Care Diagnostics market include stringent regulatory compliance, the high cost of advanced diagnostic devices, and limited access to healthcare infrastructure in rural and remote areas across the region.
03. Who are the major players in the Asia Pacific Point of Care Diagnostics Market?
Key players in the Asia Pacific Point of Care Diagnostics market include Abbott Laboratories, Roche Diagnostics, Siemens Healthineers, Danaher Corporation, and Becton, Dickinson and Company. These companies dominate the market through their extensive product portfolios and strong distribution networks.
04. What are the growth drivers of the Asia Pacific Point of Care Diagnostics Market?
Growth drivers of the Asia Pacific Point of Care Diagnostics market include the rising prevalence of chronic diseases, the adoption of telemedicine, and increased government investment in healthcare infrastructure across Asia Pacific, particularly in developing countries like India and China.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















