
Europe Pacemaker Market Outlook to 2030
Region:Europe
Author(s):Paribhasha Tiwari
Product Code:KROD5936
December 2024
81
About the Report
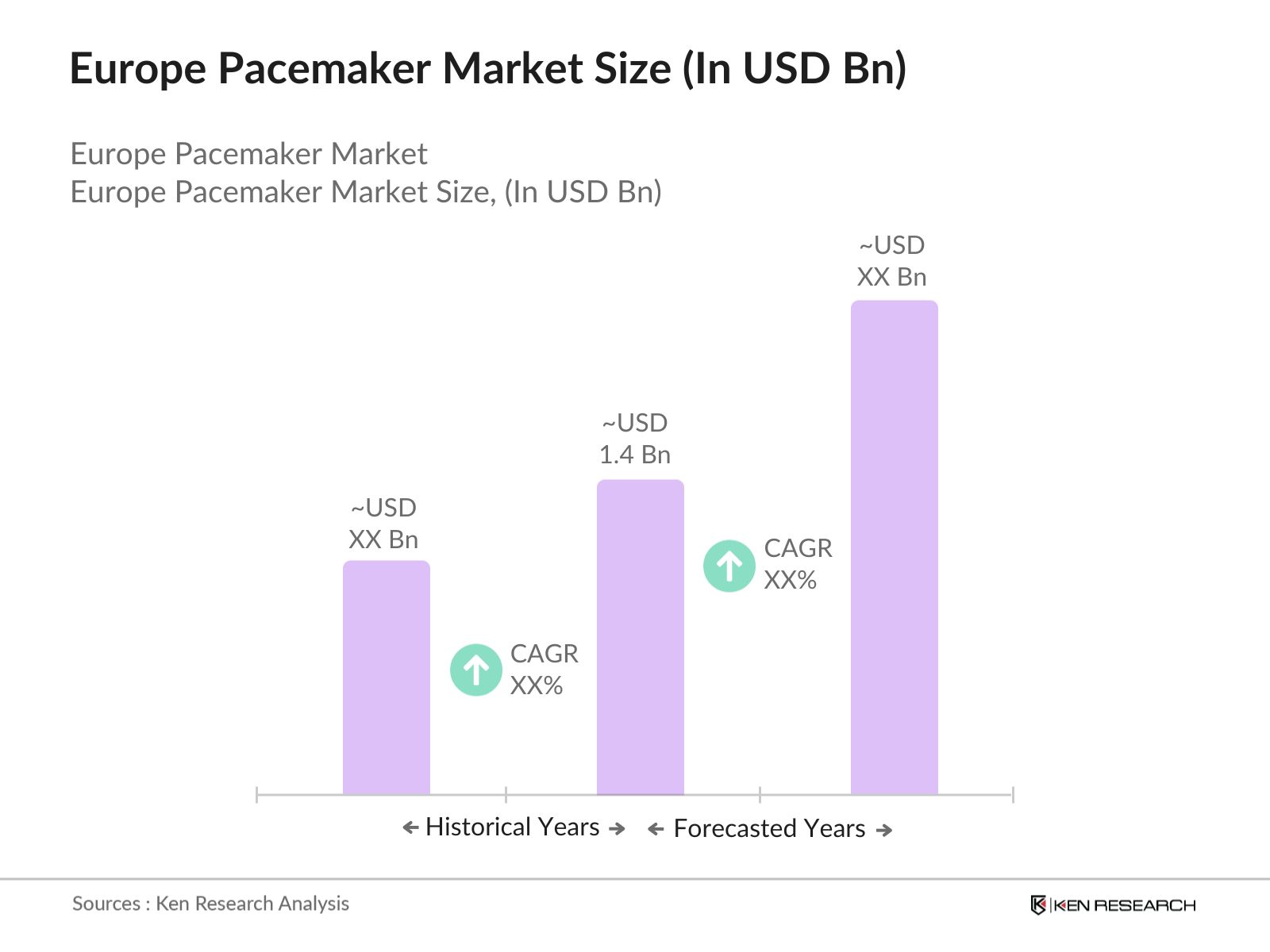
Europe Pacemaker Market Overview
- The Europe Pacemaker Market is valued at USD 1.4 billion, based on a five-year historical analysis. This market is driven by an aging population, rising cases of cardiovascular diseases, and advancements in pacemaker technology, including the adoption of leadless and MRI-compatible devices. Government healthcare expenditure and favorable reimbursement policies in several European countries further bolster the market's growth. Increasing patient awareness of modern cardiac care solutions also contributes to the market's steady expansion.
- Germany and the United Kingdom dominate the Europe Pacemaker Market due to their robust healthcare infrastructure, extensive R&D investments in cardiac care, and a high prevalence of arrhythmia cases. Germanys advanced medical facilities and strong presence of key manufacturers make it a leading country. The UK's dominance is driven by a combination of early adoption of new technologies and supportive government initiatives in healthcare innovation.
- A significant government initiative in Europe aimed at enhancing cardiac care is theEuropean Union's Horizon 2020 program, which includes funding for innovative medical technologies, including cardiac devices like pacemakers. This program, active from2014 to 2020, allocated substantial resources to support research and development in healthcare technologies, aiming to improve patient outcomes and advance medical devices.
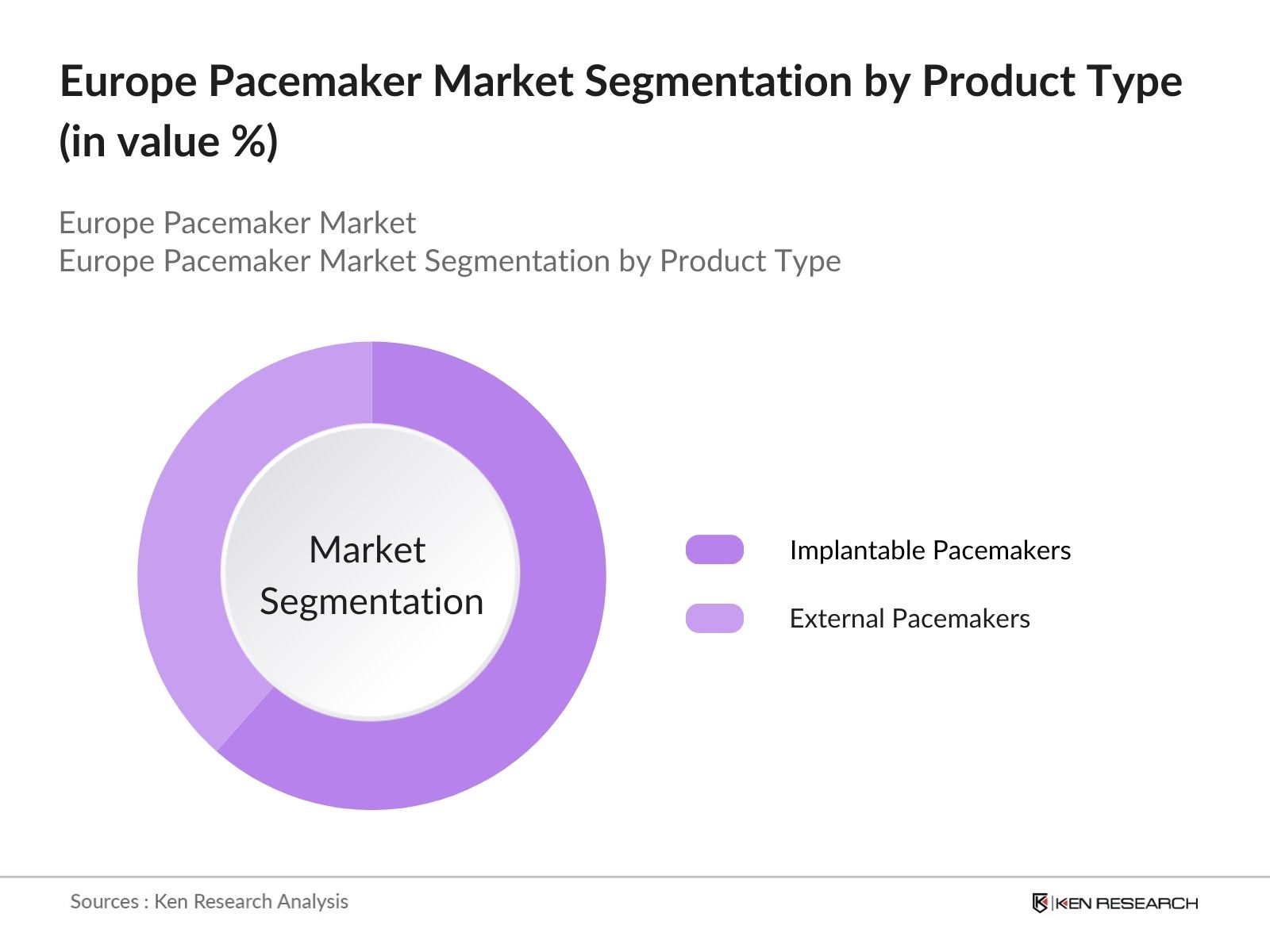
Europe Pacemaker Market Segmentation
By Product Type: The Europe Pacemaker Market is segmented by product type into implantable pacemakers and external pacemakers. Implantable pacemakers have a dominant market share due to their ability to provide long-term solutions for arrhythmias and their increasing adoption in chronic cardiovascular disease management. Innovations like leadless implantable pacemakers and MRI-compatible devices enhance their popularity.
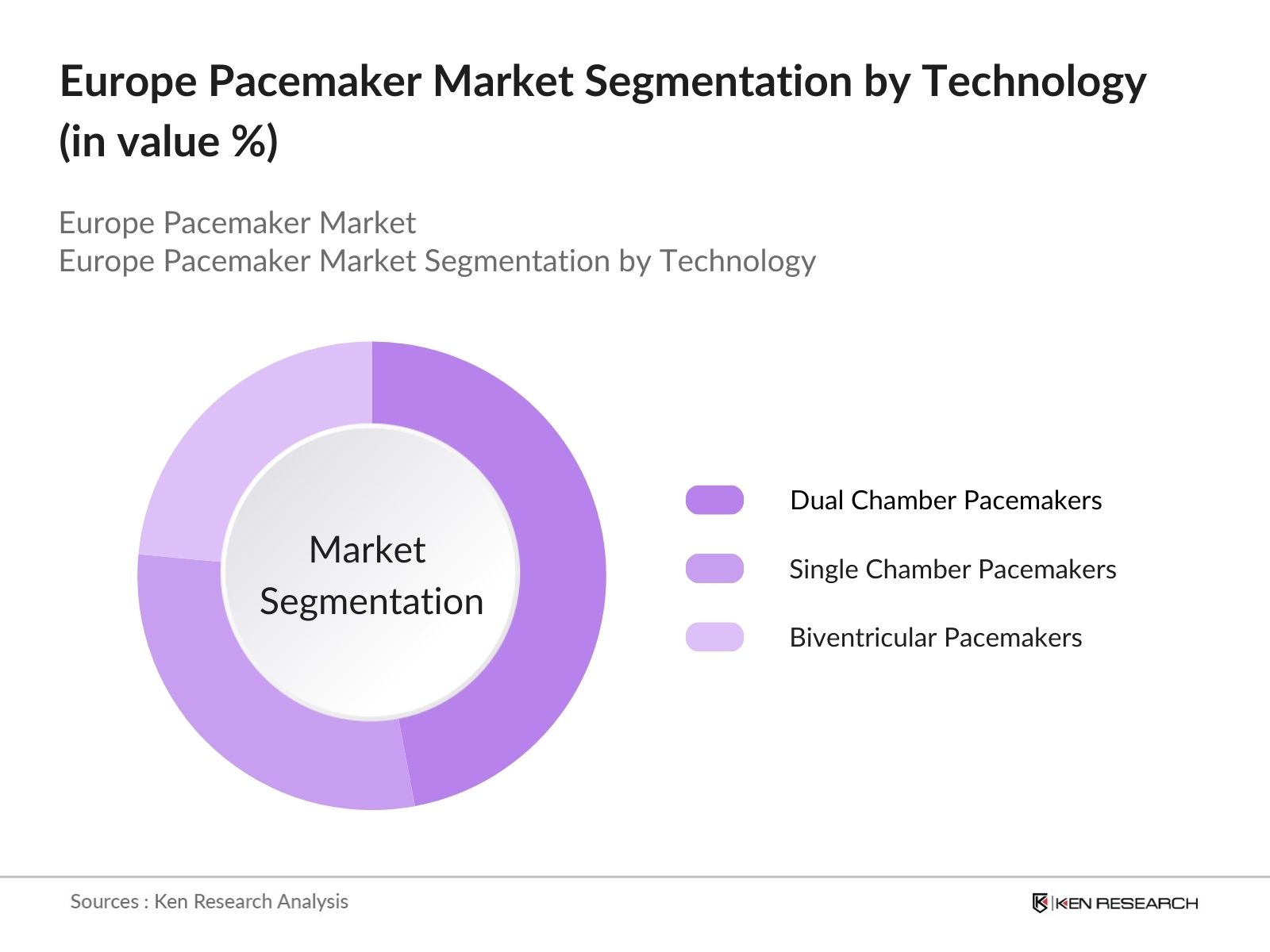
By Technology: The market is further segmented by technology into single chamber pacemakers, dual chamber pacemakers, and biventricular pacemakers. Dual chamber pacemakers hold the largest market share because they provide better synchronization of atria and ventricles, ensuring efficient cardiac output. Their extensive use in treating both bradycardia and tachycardia reinforces their dominance.
Europe Pacemaker Market Competitive Landscape
The Europe Pacemaker Market is dominated by a few key players, including Medtronic, Abbott Laboratories, and Boston Scientific Corporation. These companies leverage extensive product portfolios, innovative technologies, and global distribution networks to maintain a competitive edge. Their significant investments in R&D and partnerships with healthcare providers further solidify their leadership.
Europe Pacemaker Market Analysis
Growth Drivers
- Increasing Prevalence of Cardiovascular Diseases: The prevalence of cardiovascular diseases continues to rise, with an estimated 523 million cases globally as of 2024, driven by sedentary lifestyles and poor dietary habits. This has significantly increased the demand for pacemakers, as bradycardia and arrhythmias are common complications in such patients. In the United States alone, over 3 million people suffer from atrial fibrillation, necessitating advanced pacemaker solutions.
- Advancements in Pacemaker Technologies:Technological advancements, such as the development of leadless pacemakers and innovations in battery longevity, have transformed the industry. By 2024, approximately 500,000 leadless pacemakers were implanted globally, reducing risks associated with traditional lead-based systems. Furthermore, these innovations have streamlined implantation procedures, leading to quicker recovery times.
- Aging Population and Rising Cases of Arrhythmias: By 2024, the global population aged 65 and above has reached over 800 million, a demographic prone to arrhythmias and other cardiac disorders. In Europe alone, more than 10 million cases of arrhythmias are recorded annually, driving the need for cardiac rhythm management devices like pacemakers.
Market Challenges
- High Costs of Devices and Procedures: Pacemaker implantation procedures can cost between $30,000 and $50,000 in developed markets such as the U.S. This poses a significant financial burden for uninsured or underinsured patients, limiting access despite the availability of advanced technologies.
- Stringent Regulatory Approvals: Regulatory bodies like the U.S. FDA have strict guidelines for approving new pacemaker technologies. In 2024, over 200 applications for new pacemaker designs were under review globally, with an average approval time of two years, delaying market entry for innovative devices.
Europe Pacemaker Market Future Outlook
Over the next five years, the Europe Pacemaker Market is expected to experience significant growth due to technological advancements, increasing awareness of early diagnosis, and rising investments in healthcare infrastructure. The introduction of AI-based monitoring in pacemakers and integration with telehealth systems are anticipated to create new opportunities for market players.
Market Opportunities
- Development of MRI-Compatible Pacemakers: As of 2024, nearly 40 million MRI scans were conducted annually worldwide, many involving patients with pacemakers. The introduction of MRI-compatible devices has opened significant growth avenues, allowing patients to undergo diagnostic imaging without complications, enhancing adoption.
- Adoption of Remote Monitoring Solutions: The integration of IoT and telemedicine with pacemakers allows real-time patient monitoring. By 2024, over 1 million patients in the U.S. were utilizing remote monitoring solutions for their pacemakers, reducing hospital visits and improving long-term care outcomes.
Scope of the Report
|
By Product Type |
Implantable Pacemakers External Pacemakers |
|
By Technology |
Single Chamber Pacemakers Dual Chamber Pacemakers Biventricular Pacemakers |
|
By Application |
Arrhythmias Atrial Fibrillation Bradycardia, Tachycardia Congestive Heart Failure |
|
By End User |
Hospitals Cardiac Centers Ambulatory Surgical Centers Clinics Home Care Settings |
|
By Country |
United Kingdom Germany France Italy Spain Rest of Europe |
Products
Key Target Audience
Healthcare Providers and Hospitals
Cardiac Centers and Specialists
Medical Device Manufacturers
Distributors and Suppliers
Government and Regulatory Bodies(e.g., European Medicines Agency, National Health Services)
Insurance Companies and Reimbursement Agencies
Investors and Venture Capitalist Firms
Telemedicine and Remote Monitoring Technology Providers
Companies
Players Mentioned in the Report:
Medtronic PLC
Abbott Laboratories
Boston Scientific Corporation
BIOTRONIK SE & Co. KG
LivaNova PLC
MicroPort Scientific Corporation
Cook Medical
Zoll Medical Corporation
Pacetronix Limited
Lepu Medical Technology Co.
Table of Contents
1. Europe Pacemaker Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy
1.3 Market Growth Rate
1.4 Market Segmentation Overview
2. Europe Pacemaker Market Size (in USD Billion)
2.1 Historical Market Size
2.2 Year-On-Year Growth Analysis
2.3 Key Market Developments and Milestones
3. Europe Pacemaker Market Analysis
3.1 Growth Drivers
3.1.1 Increasing Prevalence of Cardiovascular Diseases
3.1.2 Advancements in Pacemaker Technologies
3.1.3 Aging Population and Rising Cases of Arrhythmias
3.1.4 Favorable Reimbursement Policies
3.2 Market Challenges
3.2.1 High Costs of Devices and Procedures
3.2.2 Stringent Regulatory Approvals
3.2.3 Device Malfunctions and Recalls
3.3 Opportunities
3.3.1 Development of MRI-Compatible Pacemakers
3.3.2 Adoption of Remote Monitoring Solutions
3.3.3 Growth in Emerging Markets
3.4 Trends
3.4.1 Miniaturized and Wireless Pacemakers
3.4.2 Integration with Wearable Technology
3.4.3 Increased Focus on Personalization
3.5 Regulatory Landscape
3.5.1 European Medical Device Regulation (MDR)
3.5.2 CE Marking and Compliance Standards
3.5.3 Post-Market Surveillance Requirements
3.6 SWOT Analysis
3.7 Stakeholder Ecosystem
3.8 Porter's Five Forces Analysis
3.9 Competitive Landscape
4. Europe Pacemaker Market Segmentation
4.1 By Product Type (in Value %)
4.1.1 Implantable Pacemakers
4.1.2 External Pacemakers
4.2 By Technology (in Value %)
4.2.1 Single Chamber Pacemakers
4.2.2 Dual Chamber Pacemakers
4.2.3 Biventricular Pacemakers
4.3 By Application (in Value %)
4.3.1 Arrhythmias
4.3.2 Atrial Fibrillation
4.3.3 Bradycardia
4.3.4 Tachycardia
4.3.5 Congestive Heart Failure
4.4 By End User (in Value %)
4.4.1 Hospitals
4.4.2 Cardiac Centers
4.4.3 Ambulatory Surgical Centers
4.4.4 Clinics
4.4.5 Home Care Settings
4.5 By Country (in Value %)
4.5.1 United Kingdom
4.5.2 Germany
4.5.3 France
4.5.4 Italy
4.5.5 Spain
4.5.6 Rest of Europe
5. Europe Pacemaker Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1 Medtronic PLC
5.1.2 Abbott Laboratories
5.1.3 Boston Scientific Corporation
5.1.4 BIOTRONIK SE & Co. KG
5.1.5 LivaNova PLC
5.1.6 Lepu Medical Technology Co.
5.1.7 Osypka Medical GmbH
5.1.8 Pacetronix Limited
5.1.9 MEDICO S.p.A.
5.1.10 Vitatron Holding B.V.
5.1.11 OSCOR Inc.
5.1.12 Shree Pacetronix Ltd.
5.1.13 MicroPort Scientific Corporation
5.1.14 Cook Medical
5.1.15 Zoll Medical Corporation
5.2 Cross-Comparison Parameters (Number of Employees, Headquarters, Inception Year, Revenue, Product Portfolio, Market Share, R&D Investment, Regional Presence)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers and Acquisitions
5.6 Investment Analysis
5.6.1 Venture Capital Funding
5.6.2 Government Grants
5.6.3 Private Equity Investments
6. Europe Pacemaker Market Regulatory Framework
6.1 European Medical Device Regulation (MDR) Overview
6.2 Compliance Standards for Manufacturers
6.3 Certification Processes
7. Europe Pacemaker Future Market Size (in USD Billion)
7.1 Future Market Size Projections
7.2 Key Drivers for Future Growth
8. Europe Pacemaker Future Market Segmentation
8.1 By Product Type (in Value %)
8.2 By Technology (in Value %)
8.3 By Application (in Value %)
8.4 By End User (in Value %)
8.5 By Country (in Value %)
9. Europe Pacemaker Market Analysts Recommendations
9.1 TAM/SAM/SOM Analysis
9.2 Customer Cohort Insights
9.3 Marketing and Penetration Strategies
9.4 White Space Opportunity Identification
Research Methodology
Step 1: Identification of Key Variables
The initial step involved identifying critical variables that influence the Europe Pacemaker Market, such as technological advancements, healthcare spending patterns, and patient demographics. Extensive secondary research was conducted using proprietary databases and credible publications to map the industry ecosystem.
Step 2: Market Analysis and Construction
Historical market data were compiled and analyzed to understand revenue generation, market penetration, and the adoption of different pacemaker technologies. Detailed evaluations of product categories and end-user segments ensured precise data segmentation.
Step 3: Hypothesis Validation and Expert Consultation
Primary interviews with industry experts, including cardiologists and pacemaker manufacturers, validated initial hypotheses. Insights from stakeholders helped refine market statistics and provided qualitative insights into market challenges and opportunities.
Step 4: Research Synthesis and Final Output
A comprehensive synthesis of primary and secondary data was performed. Detailed market models were created to ensure the accuracy of projections and provide an in-depth understanding of market dynamics.
Frequently Asked Questions
01. How big is the Europe Pacemaker Market?
The Europe Pacemaker Market is valued at USD 1.4 billion, driven by increasing cases of cardiovascular diseases and advancements in implantable pacemaker technology.
02. What are the challenges in the Europe Pacemaker Market?
Challenges in the Europe Pacemaker Market include high costs of devices, stringent regulatory requirements, and risks associated with device malfunctions or recalls.
03. Who are the major players in the Europe Pacemaker Market?
Major players in the Europe Pacemaker Market include Medtronic PLC, Abbott Laboratories, Boston Scientific Corporation, and BIOTRONIK SE & Co. KG, known for their strong technological integration and extensive product portfolios.
04. What drives the growth of the Europe Pacemaker Market?
The Europe Pacemaker Market is driven by an aging population, increasing prevalence of arrhythmias, and technological advancements like leadless pacemakers and remote monitoring systems.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















