
Global Cancer Diagnostics Market Outlook to 2030
Region:Global
Author(s):Vijay Kumar
Product Code:KROD3750
December 2024
98
About the Report
Global Cancer Diagnostics Market Overview
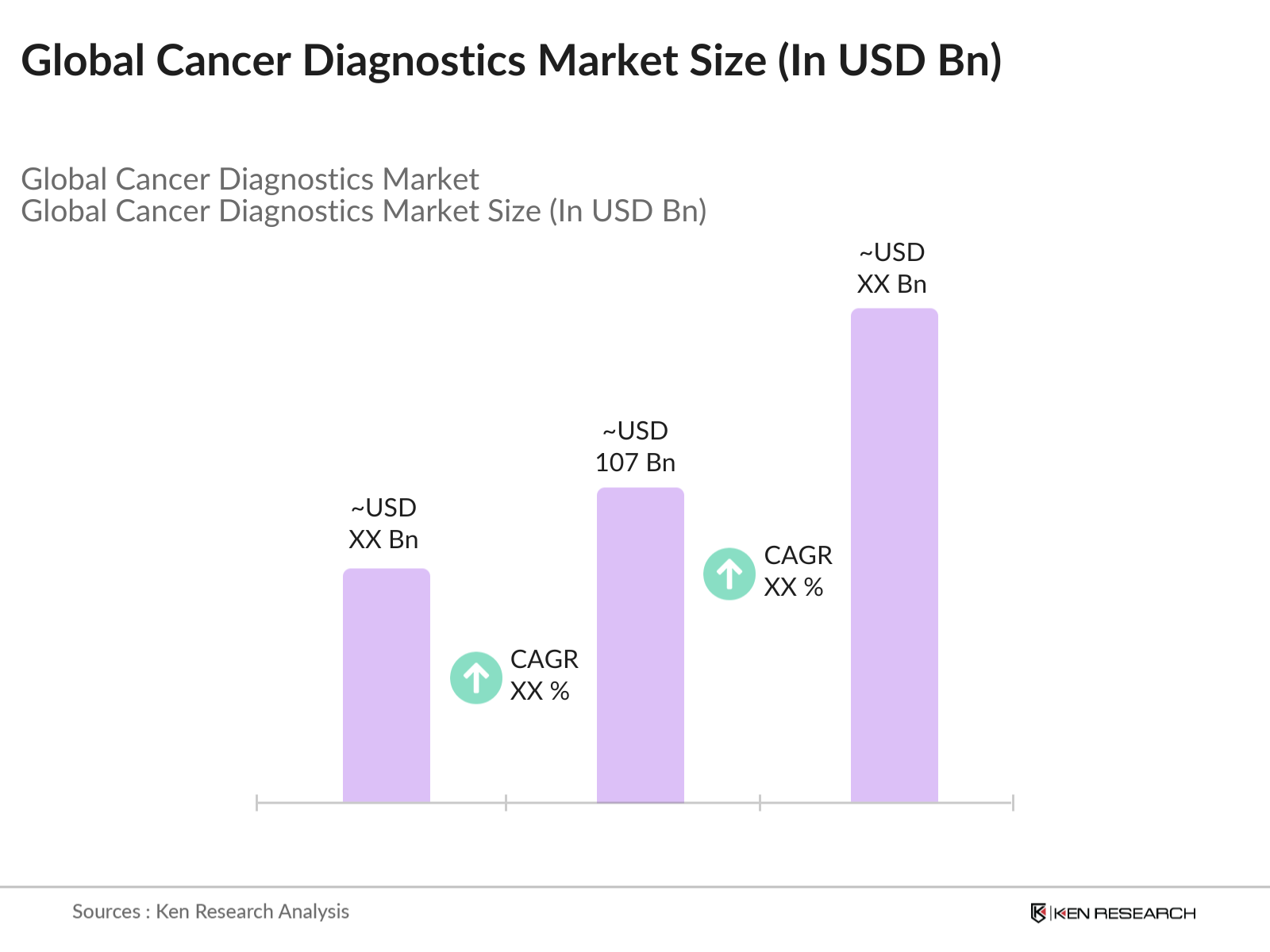
- The global cancer diagnostics market is valued at USD 107 billion, based on a detailed analysis of historical market data. This market is primarily driven by the rising incidence of cancer worldwide and the adoption of advanced diagnostic technologies such as liquid biopsies, next-generation sequencing (NGS), and molecular diagnostics. The increased emphasis on early detection and personalized treatment solutions is fueling demand for cancer diagnostics across the healthcare industry.
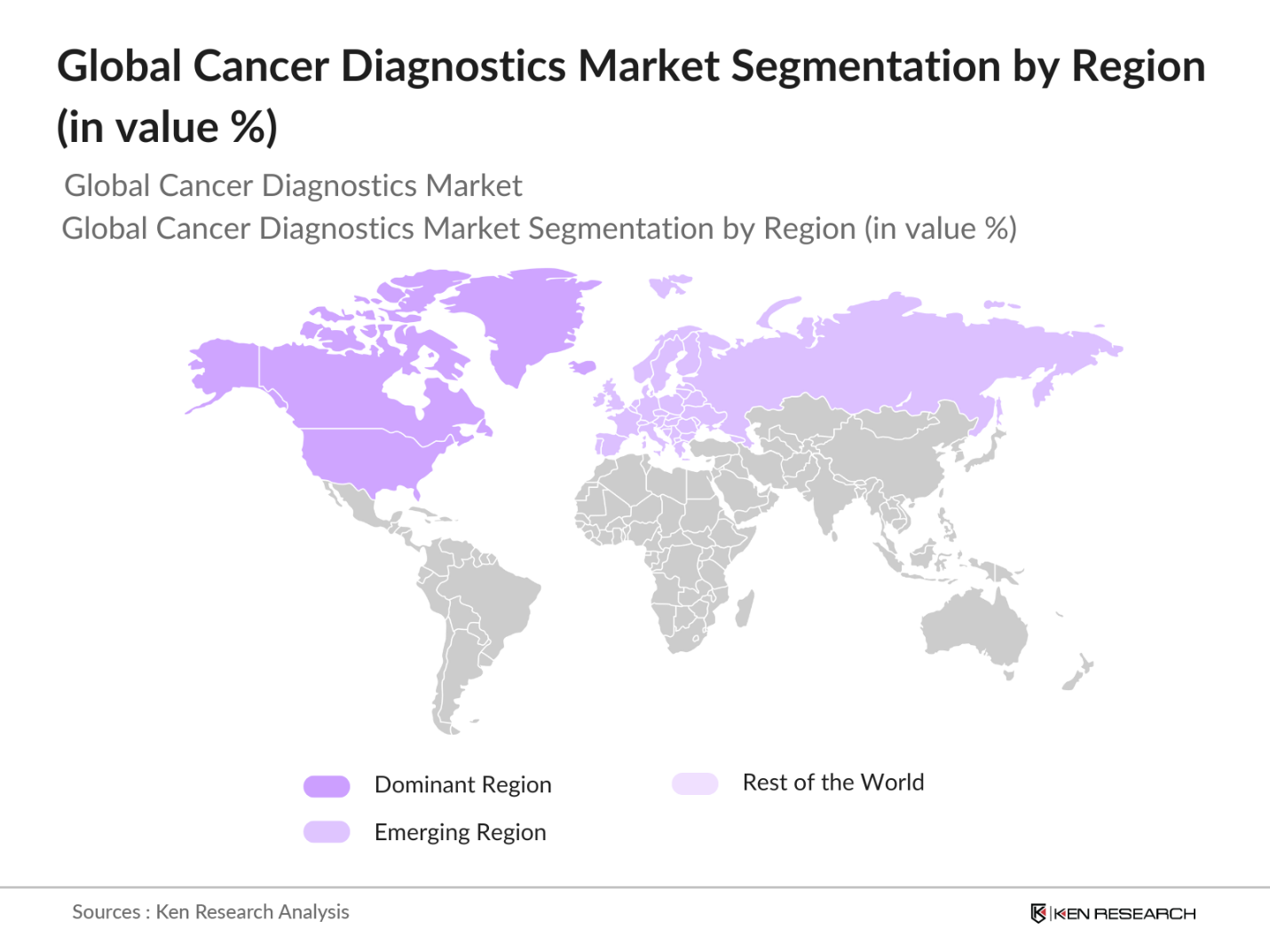
- The global cancer diagnostics market is dominated by North America, followed by Europe and the Asia-Pacific (APAC) region. North America holds a leading position due to its advanced healthcare infrastructure, high adoption rates of innovative diagnostic techniques, and extensive research and development activities. The United States is a major player within this region, attributed to a well-established network of diagnostic laboratories and a high prevalence of cancer cases.
- The cancer diagnostics market is regulated by various regional bodies such as the FDA in the US and EMA in Europe. These organizations establish the standards and protocols for diagnostic device approval, ensuring safety and efficacy. Compliance with these standards is crucial for market entry but poses challenges due to varying regulatory requirements across regions. Companies must navigate complex approval pathways to bring new diagnostic products to market, which can delay product launches.
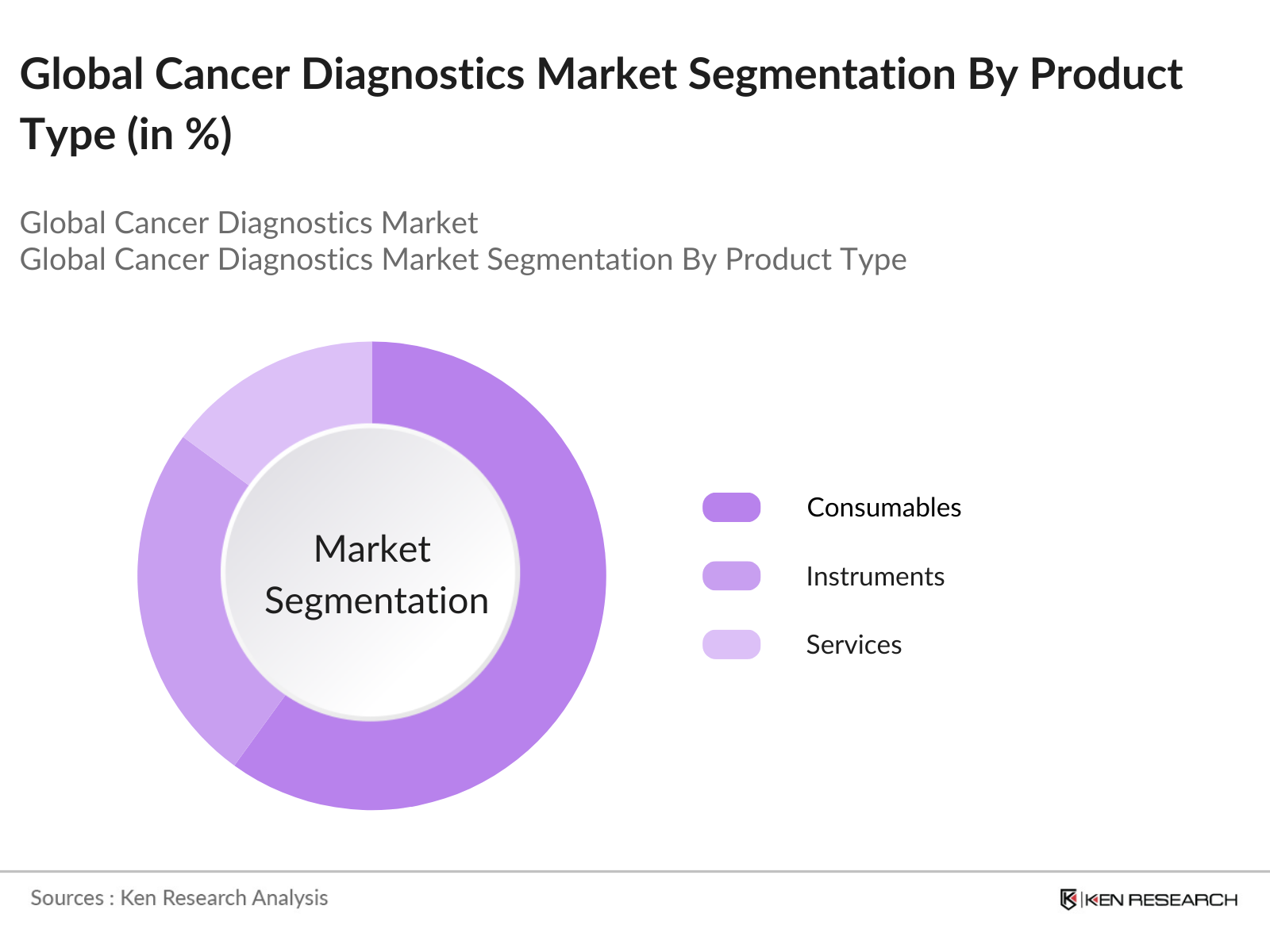
Global Cancer Diagnostics Market Segmentation
By Product Type: The cancer diagnostics market is segmented into consumables, instruments, and services. Consumables, such as antibodies, reagents, and probes, hold the dominant market share due to their recurrent use in diagnostic testing. Consumables are crucial for diagnostic laboratories as they enable continuous testing, driving their demand. The ease of availability and advancements in reagent formulations have further cemented the dominance of this segment.
By Technology: The cancer diagnostics market is categorized by technology into Polymerase Chain Reaction (PCR), Immunohistochemistry (IHC), In Situ Hybridization (ISH), Next-Generation Sequencing (NGS), and Flow Cytometry. PCR has emerged as a leading technology segment, driven by its high sensitivity, specificity, and ability to detect minimal residual disease. PCR's widespread application in both clinical and research settings has led to its dominance.
By Region: The cancer diagnostics market is segmented by region into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America holds the largest market share, primarily due to the high incidence of cancer, availability of advanced diagnostic infrastructure, and substantial investments in R&D. Asia Pacific is the fastest-growing region, driven by increasing healthcare expenditure, rising awareness about early cancer detection, and the growing adoption of molecular diagnostics.
Global Cancer Diagnostics Market Competitive Landscape
The global cancer diagnostics market is characterized by the presence of established players who dominate due to extensive product portfolios and strong R&D capabilities. Major companies like Abbott Laboratories, Roche Holding AG, and Thermo Fisher Scientific Inc. have a strong foothold in the market, supported by continuous product innovations and strategic collaborations.
Global Cancer Diagnostics Industry Analysis
Growth Drivers
- Increasing Cancer Prevalence and Incidence Rates (e.g., breast, lung, colorectal): The global burden of cancer has been rising significantly. In 2022, there were an estimated 20 million new cancer cases and 9.7 million cancer-related deaths worldwide, according to the World Health Organization (WHO). This increase is driven by factors such as an aging population and high prevalence of risk factors like tobacco use. Lung cancer had the highest number of new cases at 2.5 million, followed by breast cancer at 2.3 million, and colorectal cancer at 1.9 million.
- Rising Adoption of Precision Medicine and Personalized Oncology Treatments: Precision medicine and personalized treatments have seen increased adoption, particularly in cancer care. Advances in genomic profiling and next-generation sequencing (NGS) allow for more accurate targeting of cancer therapies. This has become a crucial aspect of cancer treatment, enabling tailored therapeutic approaches for over 53 million people alive within five years of their cancer diagnosis in 2022.
- Technological Advancements in Diagnostic Platforms (e.g., Next-Generation Sequencing, Liquid Biopsies): Technological innovations such as next-generation sequencing (NGS) and liquid biopsies are transforming cancer diagnostics. NGS, which enables comprehensive genomic profiling, and liquid biopsies, which provide non-invasive analysis of cancer biomarkers, are gaining widespread clinical adoption. In 2022, approximately 1.5 million prostate cancer and 970,000 stomach cancer cases were diagnosed using such advanced diagnostic technologies.
Market Challenges
- High Cost of Diagnostic Instruments and Limited Access in Low-Income Regions: Access to advanced cancer diagnostic tools remains limited in low-income regions due to the high cost of instruments and inadequate healthcare infrastructure. According to WHO, while high-income countries report improved cancer survival rates, only 28% of countries globally provide comprehensive cancer care, including pain relief and palliative care.
- Regulatory and Reimbursement Challenges (Complex Approval Processes): The complex regulatory environment and reimbursement challenges present a major hurdle for cancer diagnostics. Stringent approval processes by regulatory bodies like the FDA and EMA often delay the introduction of new diagnostic tools in the market. For example, compliance with varying regional standards makes it difficult for companies to achieve simultaneous global market entry, impacting the availability of innovative diagnostic solutions for patients.
Global Cancer Diagnostics Market Future Outlook
Over the next few years, the global cancer diagnostics market is expected to witness substantial growth due to the rising incidence of cancer, technological advancements, and increasing investments in research and development. The adoption of non-invasive diagnostic methods, such as liquid biopsies and NGS, is projected to reshape the market landscape.
Market Opportunities
- Expanding Applications of Artificial Intelligence in Diagnostic Imaging: Artificial intelligence (AI) is playing a transformative role in cancer diagnostics. AI-driven tools for image analysis can detect cancerous patterns with high accuracy and speed, assisting radiologists in early diagnosis. These tools are particularly beneficial in resource-limited settings, enabling remote diagnostic support. AI integration in diagnostic imaging is becoming more prevalent, creating significant opportunities for companies to develop AI-enabled diagnostics that can support early and accurate detection of various cancer types.
- Strategic Collaborations and Acquisitions in the Cancer Profiling Space: The market is witnessing increased strategic collaborations and acquisitions aimed at strengthening cancer profiling capabilities. Partnerships between pharmaceutical companies and diagnostic firms are enabling the development of integrated diagnostic and therapeutic solutions, enhancing personalized medicine. Such collaborations not only facilitate technology sharing but also accelerate the commercialization of novel diagnostics.
Scope of the Report
|
Product |
Consumables (Kits, Reagents, Antibodies, Probes) Instruments (Imaging Instruments, Pathology Instruments, Molecular Diagnostics Instruments) Services (Oncology Testing, Imaging Services) |
|
Technology |
PCR IHC ISH NGS Flow Cytometry |
|
Application |
Breast Cancer Diagnostics Lung Cancer Diagnostics Colorectal Cancer Diagnostics Prostate Cancer Diagnostics Other Cancers |
|
End User |
Hospitals & Clinics Diagnostic Laboratories Research Institutes Others (Point-of-Care Settings) |
|
Region |
North America (U.S., Canada, Mexico) Europe (Germany, France, UK, Italy, Spain) Asia Pacific (China, Japan, India, South Korea) Latin America (Brazil, Argentina) Middle East & Africa (South Africa, GCC) |
Products
Key Target Audience
Investors and Venture Capital Firms
Diagnostic Instrument Manufacturers
Consumables Suppliers
Hospitals and Healthcare Providers
Cancer Research Institutes
Government and Regulatory Bodies (e.g., FDA, EMA)
Insurance and Reimbursement Organizations
Biotechnology and Pharmaceutical Companies
Companies
Players Mentioned in the Report
Abbott Laboratories
Roche Holding AG
Thermo Fisher Scientific Inc.
Siemens Healthineers AG
Illumina Inc.
Qiagen NV
Becton Dickinson & Co.
Koninklijke Philips NV
Hologic Inc.
GE HealthCare Technologies Inc.
PerkinElmer Inc.
Table of Contents
1. Global Cancer Diagnostics Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy
1.3 Market Dynamics and Growth Indicators
1.4 Market Segmentation Overview
2. Global Cancer Diagnostics Market Size (USD Billion)
2.1 Historical Market Size Analysis
2.2 Year-on-Year Growth Analysis
2.3 Key Market Developments and Milestones
3. Global Cancer Diagnostics Market Analysis
3.1 Growth Drivers
3.1.1 Increasing Cancer Prevalence and Incidence Rates (e.g., breast, lung, colorectal)
3.1.2 Rising Adoption of Precision Medicine and Personalized Oncology Treatments
3.1.3 Technological Advancements in Diagnostic Platforms (e.g., Next-Generation Sequencing, Liquid Biopsies)
3.1.4 Growing Investments in Research and Development by Leading Players
3.2 Market Challenges
3.2.1 High Cost of Diagnostic Instruments and Limited Access in Low-Income Regions
3.2.2 Regulatory and Reimbursement Challenges (Complex approval processes)
3.2.3 Limited Sensitivity and Specificity in Early-Stage Detection
3.3 Opportunities
3.3.1 Expanding Applications of Artificial Intelligence in Diagnostic Imaging
3.3.2 Strategic Collaborations and Acquisitions in the Cancer Profiling Space
3.3.3 Rising Demand for Non-Invasive Diagnostic Techniques
3.4 Market Trends
3.4.1 Increased Utilization of Biomarker-Based Diagnostics
3.4.2 Emergence of Multi-Cancer Early Detection (MCED) Tests
3.4.3 Shift Towards At-Home and Point-of-Care Diagnostic Solutions
3.5 Regulatory Landscape
3.5.1 Regional Regulatory Bodies (e.g., FDA, EMA) and Compliance Standards
3.5.2 Impact of Regulatory Changes on Market Dynamics
3.6 SWOT Analysis
3.7 Value Chain Analysis
3.8 Porters Five Forces Analysis
4. Global Cancer Diagnostics Market Segmentation
4.1 By Product Type (Value %)
4.1.1 Consumables (Kits, Reagents, Antibodies, Probes)
4.1.2 Instruments (Imaging Instruments, Pathology Instruments, Molecular Diagnostics Instruments)
4.1.3 Services (Oncology Testing, Imaging Services)
4.2 By Technology (Value %)
4.2.1 Polymerase Chain Reaction (PCR)
4.2.2 Immunohistochemistry (IHC)
4.2.3 In Situ Hybridization (ISH)
4.2.4 Next-Generation Sequencing (NGS)
4.2.5 Flow Cytometry
4.3 By Application (Value %)
4.3.1 Breast Cancer Diagnostics
4.3.2 Lung Cancer Diagnostics
4.3.3 Colorectal Cancer Diagnostics
4.3.4 Prostate Cancer Diagnostics
4.3.5 Other Cancers
4.4 By End User (Value %)
4.4.1 Hospitals & Clinics
4.4.2 Diagnostic Laboratories
4.4.3 Research Institutes
4.4.4 Others (Point-of-Care Settings)
4.5 By Region (Value %)
4.5.1 North America (U.S., Canada, Mexico)
4.5.2 Europe (Germany, France, UK, Italy, Spain)
4.5.3 Asia Pacific (China, Japan, India, South Korea)
4.5.4 Latin America (Brazil, Argentina)
4.5.5 Middle East & Africa (South Africa, GCC)
5. Global Cancer Diagnostics Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1 Abbott Laboratories
5.1.2 Roche Holding AG
5.1.3 GE HealthCare Technologies Inc.
5.1.4 Qiagen NV
5.1.5 Becton Dickinson & Co.
5.1.6 Koninklijke Philips NV
5.1.7 Siemens Healthineers AG
5.1.8 Thermo Fisher Scientific Inc.
5.1.9 Illumina Inc.
5.1.10 Hologic Inc.
5.1.11 Exact Sciences Corporation
5.1.12 Bio-Rad Laboratories
5.1.13 Agilent Technologies
5.1.14 PerkinElmer Inc.
5.1.15 NeoGenomics Laboratories
5.2 Cross Comparison Parameters
5.2.1 Revenue
5.2.2 R&D Expenditure
5.2.3 Product Portfolio
5.2.4 Strategic Initiatives (Mergers, Acquisitions, Collaborations)
5.2.5 Market Share Analysis
5.2.6 Number of Employees
5.2.7 Global Presence
5.3 Strategic Initiatives
5.4 Mergers and Acquisitions
5.5 Investment Analysis
5.6 Government and Private Sector Funding
6. Global Cancer Diagnostics Market Regulatory Framework
6.1 Regulatory Standards for Diagnostic Instruments and Kits
6.2 Compliance and Certification Processes
6.3 Impact of Regulatory Changes on Market Access
7. Global Cancer Diagnostics Market Future Outlook (USD Billion)
7.1 Projected Market Size and Growth Potential
7.2 Factors Influencing Future Market Trends
8. Global Cancer Diagnostics Market Segmentation
8.1 By Product Type (Value %)
8.2 By Technology (Value %)
8.3 By Application (Value %)
8.4 By End User (Value %)
8.5 By Region (Value %)
9. Global Cancer Diagnostics Market Analysts Recommendations
9.1 Market Expansion Strategies
9.2 Target Market Segments for Investment
9.3 Key Differentiators for Competitive Advantage
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The first phase involves constructing an ecosystem map encompassing all major stakeholders within the global cancer diagnostics market. This step is underpinned by extensive desk research, utilizing a combination of secondary and proprietary databases to gather comprehensive industry-level information. The primary objective is to identify and define the critical variables that influence market dynamics.
Step 2: Market Analysis and Construction
In this phase, we compile and analyze historical data pertaining to the global cancer diagnostics market. This includes assessing market penetration, the ratio of diagnostic providers to healthcare institutions, and the resultant revenue generation. An evaluation of service quality statistics is also conducted to ensure the reliability and accuracy of the revenue estimates.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are developed and subsequently validated through computer-assisted telephone interviews (CATIs) with industry experts representing a diverse array of companies. These consultations provide valuable operational and financial insights directly from industry practitioners, which are instrumental in refining and corroborating the market data.
Step 4: Research Synthesis and Final Output
The final phase involves direct engagement with multiple cancer diagnostic companies to acquire detailed insights into product segments, sales performance, consumer preferences, and other pertinent factors. This interaction serves to verify and complement the statistics derived from the bottom-up approach, thereby ensuring a comprehensive, accurate, and validated analysis of the global cancer diagnostics market.
Frequently Asked Questions
01. How big is the Global Cancer Diagnostics Market?
The global cancer diagnostics market is valued at USD 107 million, driven by increased demand for early cancer detection and technological advancements in diagnostic tools.
02. What are the major challenges in the Global Cancer Diagnostics Market?
Major challenges include high costs associated with advanced diagnostic techniques, stringent regulatory frameworks, and the limited availability of diagnostic infrastructure in low-income regions.
03. Who are the major players in the Global Cancer Diagnostics Market?
Key players in the market include Abbott Laboratories, Roche Holding AG, Thermo Fisher Scientific Inc., Siemens Healthineers AG, and Illumina Inc. These companies dominate due to their extensive product portfolios and strong R&D investments.
04. What are the growth drivers of the Global Cancer Diagnostics Market?
The market is propelled by factors such as the rising prevalence of cancer, growing adoption of precision medicine, and increasing investments in research and development for innovative diagnostic technologies.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















