Region:Global
Author(s):Geetanshi
Product Code:KRAD0078
Pages:82
Published On:August 2025
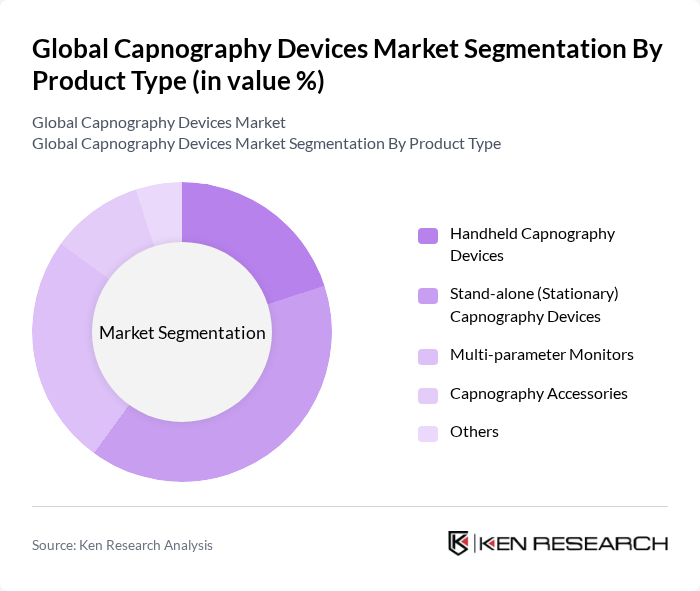
By Product Type:The product type segmentation includes handheld capnography devices, stand-alone (stationary) capnography devices, multi-parameter monitors, capnography accessories, and others. Among these, stand-alone capnography devices are leading the market due to their reliability and accuracy in clinical settings. The increasing demand for continuous monitoring in hospitals and emergency medical services has further solidified their position as the preferred choice for healthcare professionals. Handheld devices are gaining traction due to their portability and use in ambulatory and home care settings.
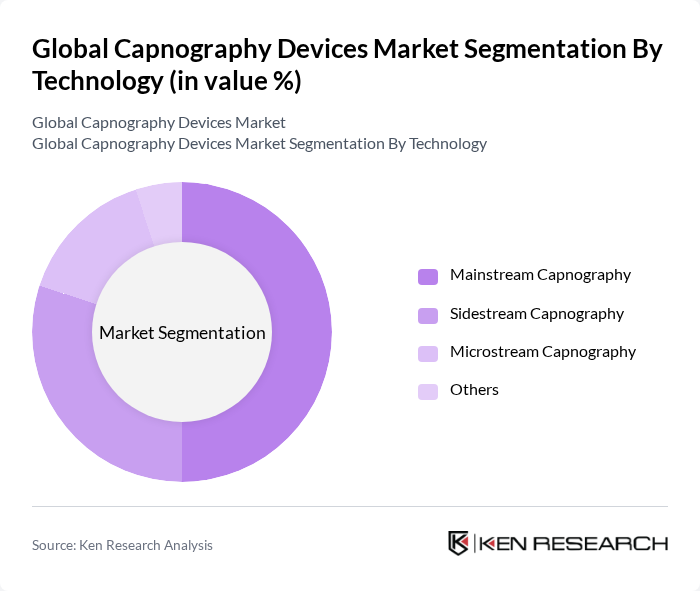
By Technology:The technology segmentation encompasses mainstream capnography, sidestream capnography, microstream capnography, and others. Mainstream capnography remains the dominant technology in the market, favored for its real-time monitoring capabilities and accuracy. This technology is particularly beneficial in critical care settings where immediate feedback on a patient's respiratory status is crucial for effective management. Sidestream and microstream technologies are increasingly adopted for their versatility in different clinical environments.
The Global Capnography Devices Market is characterized by a dynamic mix of regional and international players. Leading participants such as Medtronic plc, Koninklijke Philips N.V. (Philips Healthcare), Masimo Corporation, Smiths Medical (ICU Medical, Inc.), Nihon Kohden Corporation, Drägerwerk AG & Co. KGaA, GE Healthcare, Nonin Medical, Inc., Welch Allyn (Hillrom, now part of Baxter International Inc.), ZOLL Medical Corporation (Asahi Kasei Group), Intersurgical Ltd., Edan Instruments, Inc., Ambu A/S, Diamedica (UK) Limited, BD (Becton, Dickinson and Company) contribute to innovation, geographic expansion, and service delivery in this space.
The future of the capnography devices market in future appears promising, driven by increasing healthcare investments and a shift towards home healthcare solutions. As the population ages and the prevalence of respiratory diseases rises, the demand for effective monitoring technologies will continue to grow. Additionally, the integration of capnography with telemedicine is expected to enhance patient care, allowing for remote monitoring and timely interventions. These trends indicate a robust market trajectory, fostering innovation and accessibility in respiratory care.
| Segment | Sub-Segments |
|---|---|
| By Product Type | Handheld Capnography Devices Stand-alone (Stationary) Capnography Devices Multi-parameter Monitors Capnography Accessories Others |
| By Technology | Mainstream Capnography Sidestream Capnography Microstream Capnography Others |
| By Application | Procedural Sedation Emergency Medicine Critical Care Monitoring Pain Management Respiratory Monitoring Sleep Apnea Monitoring Others |
| By End-User | Hospitals Ambulatory Surgical Centers Home Care Settings Emergency Medical Services Clinics Others |
| By Distribution Channel | Direct Sales Distributors Online Sales Retail Pharmacies Others |
| By Region | North America Europe Asia-Pacific Latin America Middle East & Africa |
| By Price Range | Low Price Range Mid Price Range High Price Range |
| Scope Item/Segment | Sample Size | Target Respondent Profiles |
|---|---|---|
| Hospital Capnography Device Usage | 100 | Anesthesiologists, Critical Care Physicians |
| Home Healthcare Capnography Applications | 60 | Home Care Nurses, Respiratory Therapists |
| Emergency Medical Services (EMS) Capnography Practices | 50 | Paramedics, EMS Directors |
| Capnography Device Procurement in Healthcare Facilities | 70 | Procurement Managers, Hospital Administrators |
| Research and Development in Capnography Technology | 40 | Biomedical Engineers, Product Development Managers |
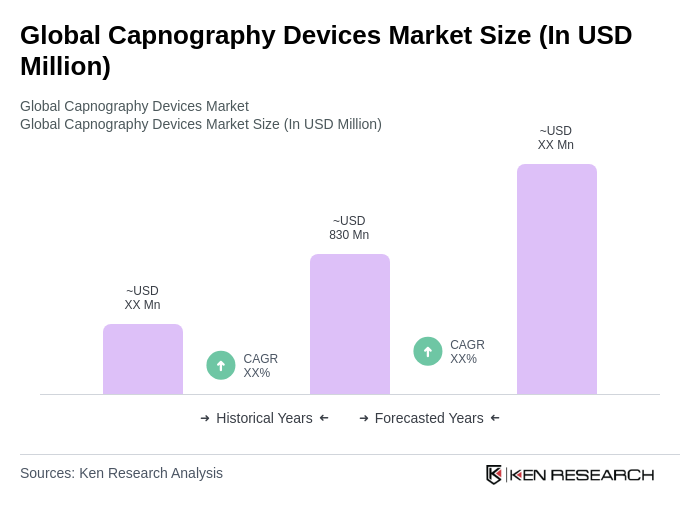
The Global Capnography Devices Market is valued at approximately USD 830 million, driven by factors such as the increasing prevalence of respiratory diseases, advancements in technology, and a growing focus on patient safety in clinical settings.