Global Castrate Resistant Prostate Cancer Therapeutics Market Overview
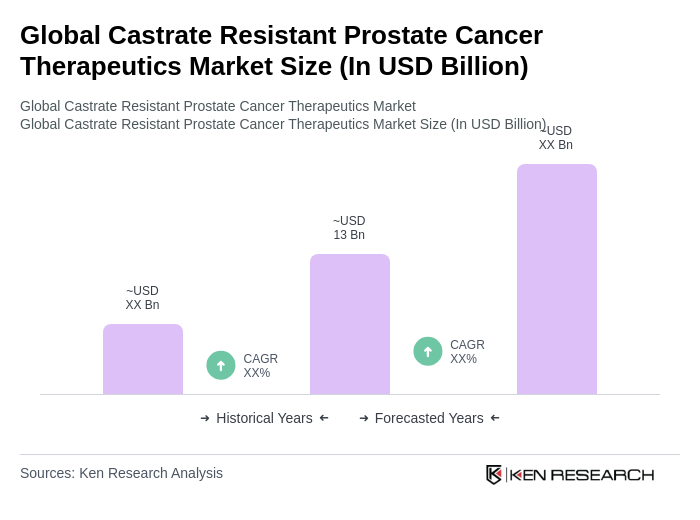
- The Global Castrate Resistant Prostate Cancer Therapeutics Market is valued at USD 13 billion, based on a five-year historical analysis. This growth is primarily driven by the increasing prevalence of prostate cancer, advancements in treatment options, and a growing aging population. The demand for innovative therapies, particularly next-generation hormonal therapies and immunotherapies, has significantly contributed to the market's expansion.
- Key players in this market are predominantly located in North America and Europe, with the United States and Germany leading due to their robust healthcare infrastructure, high investment in research and development, and a strong presence of pharmaceutical companies. These regions benefit from advanced medical technologies and a higher rate of diagnosis and treatment of prostate cancer.
- In recent years, the U.S. Food and Drug Administration (FDA) has implemented regulatory initiatives to expedite the approval process for innovative prostate cancer therapies. These include priority review pathways for drugs that demonstrate significant improvements over existing treatments, enhancing patient access to advanced therapies and fostering competition among pharmaceutical companies.
Global Castrate Resistant Prostate Cancer Therapeutics Market Segmentation
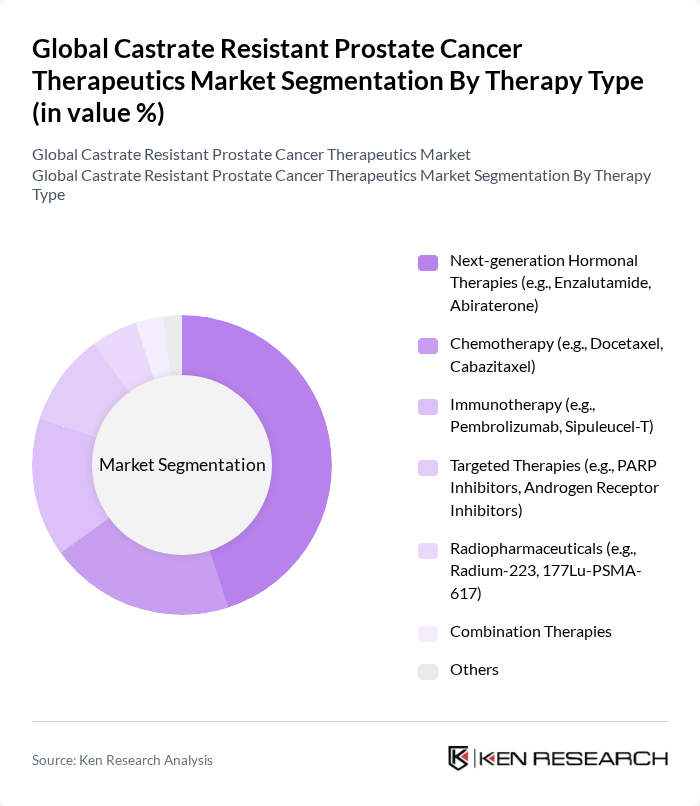
By Therapy Type:The market is segmented into various therapy types, including Next-generation Hormonal Therapies, Chemotherapy, Immunotherapy, Targeted Therapies, Radiopharmaceuticals, Combination Therapies, and Others. Among these, Next-generation Hormonal Therapies, such as Enzalutamide and Abiraterone, dominate the market due to their effectiveness in prolonging survival and improving quality of life for patients. The increasing adoption of these therapies is driven by their favorable clinical outcomes and the growing awareness among healthcare providers and patients.
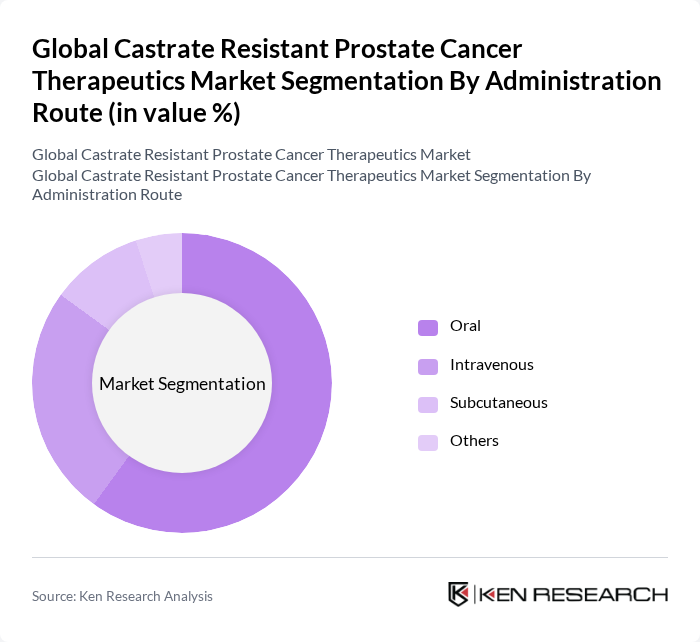
By Administration Route:The administration routes for therapies include Oral, Intravenous, Subcutaneous, and Others. Oral administration is the most preferred route due to its convenience and ease of use, allowing patients to manage their treatment regimens at home. This preference is further supported by the development of effective oral formulations that enhance patient compliance and satisfaction.
Global Castrate Resistant Prostate Cancer Therapeutics Market Competitive Landscape
The Global Castrate Resistant Prostate Cancer Therapeutics Market is characterized by a dynamic mix of regional and international players. Leading participants such as Johnson & Johnson (Janssen Pharmaceuticals), Pfizer Inc., Astellas Pharma Inc., Bayer AG, Sanofi S.A., AstraZeneca PLC, Novartis AG, Merck & Co., Inc., Bristol-Myers Squibb Company, Eli Lilly and Company, Ipsen S.A., Clovis Oncology, Inc., Takeda Pharmaceutical Company Limited, Endo International plc, Spectrum Pharmaceuticals, Inc., Myovant Sciences Ltd., Tolmar Pharmaceuticals, Inc., Dendreon Pharmaceuticals LLC contribute to innovation, geographic expansion, and service delivery in this space.
Global Castrate Resistant Prostate Cancer Therapeutics Market Industry Analysis
Growth Drivers
- Increasing Prevalence of Prostate Cancer:The incidence of prostate cancer is projected to reach approximately 1.4 million new cases globally in the future, according to the World Health Organization. This rising prevalence is primarily attributed to aging populations, with men over 65 years accounting for nearly 60% of cases. As awareness increases, more individuals are diagnosed, driving demand for effective therapeutics. This trend is particularly evident in regions with aging demographics, such as North America and Europe, where healthcare systems are adapting to manage this growing patient population.
- Advancements in Therapeutic Options:Significant advancements in treatment modalities, including novel androgen receptor inhibitors and immunotherapies, are transforming the landscape of castrate-resistant prostate cancer (CRPC). For instance, the introduction of drugs like enzalutamide and abiraterone has shown improved survival rates, with clinical trials indicating a 30% reduction in mortality. These innovations are supported by a robust pipeline of over 50 investigational drugs, reflecting a commitment to enhancing patient outcomes and expanding treatment options in the CRPC market.
- Increased Funding for Cancer Research:Global funding for cancer research is expected to exceed $200 billion in the future, driven by both public and private sectors. This influx of capital is facilitating the development of new therapies and clinical trials, particularly in prostate cancer. For example, the National Cancer Institute allocated approximately $5 billion in the future for prostate cancer research, fostering innovation and accelerating the introduction of cutting-edge treatments. This financial support is crucial for advancing therapeutic options and improving patient care in the CRPC market.
Market Challenges
- High Treatment Costs:The financial burden of CRPC therapies remains a significant challenge, with annual treatment costs exceeding $100,000 for some advanced therapies. This high cost can limit patient access, particularly in low- and middle-income countries where healthcare budgets are constrained. As a result, many patients may forgo necessary treatments, leading to poorer health outcomes and increased mortality rates. Addressing these financial barriers is essential for improving access to life-saving therapies in the CRPC market.
- Stringent Regulatory Approvals:The regulatory landscape for new cancer therapies is increasingly complex, with agencies like the FDA and EMA imposing rigorous approval processes. In the future, the average time for drug approval is approximately 10 months, which can delay patient access to innovative treatments. Additionally, the need for extensive clinical trials often results in high costs and resource allocation challenges for pharmaceutical companies. Navigating these regulatory hurdles is critical for timely market entry of new CRPC therapies.
Global Castrate Resistant Prostate Cancer Therapeutics Market Future Outlook
The future of the CRPC therapeutics market is poised for significant transformation, driven by ongoing innovations in treatment strategies and a growing emphasis on personalized medicine. As the understanding of prostate cancer biology deepens, tailored therapies are expected to emerge, enhancing treatment efficacy. Furthermore, the integration of digital health solutions and telemedicine is likely to improve patient monitoring and adherence, ultimately leading to better health outcomes. These trends indicate a dynamic and evolving market landscape that prioritizes patient-centric approaches.
Market Opportunities
- Development of Personalized Medicine:The shift towards personalized medicine presents a significant opportunity in the CRPC market. By leveraging genomic profiling, therapies can be tailored to individual patient needs, potentially improving treatment efficacy. This approach is supported by a growing body of research, with over 30% of clinical trials focusing on biomarker-driven therapies, indicating a robust trend towards customization in cancer treatment.
- Expansion into Emerging Markets:Emerging markets, particularly in Asia-Pacific and Latin America, represent a substantial growth opportunity for CRPC therapeutics. With prostate cancer incidence rising in these regions, coupled with increasing healthcare investments, companies can tap into new patient populations. The Asia-Pacific region alone is expected to see a 25% increase in prostate cancer cases in the future, highlighting the potential for market expansion and increased access to innovative therapies.