
Global Decentralized Clinical Trials Market Outlook to 2030
Region:Global
Author(s):Naman Rohilla
Product Code:KROD9661
December 2024
80
About the Report
Global Decentralized Clinical Trials Market Overview
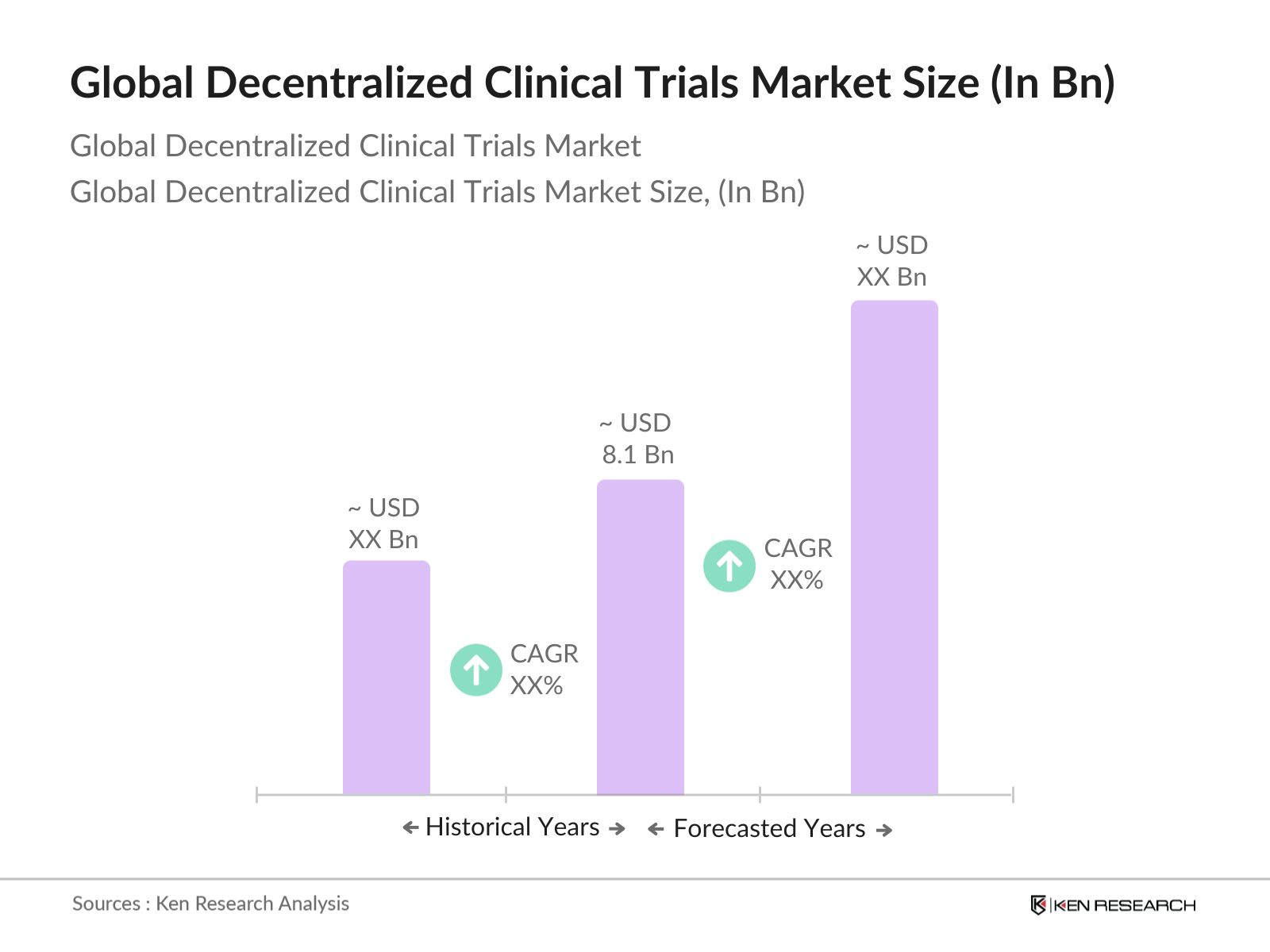
- The global Decentralized Clinical Trials (DCT) market, valued at USD 8.1 billion, is primarily driven by the rapid adoption of digital health technologies and the rising demand for patient-centric trials. The need to reach diverse patient populations, especially those with limited access to traditional clinical trial sites, has fueled this growth. Furthermore, DCTs are instrumental in reducing the logistical and operational burdens associated with conventional trials, making them a popular choice among biopharmaceutical companies and contract research organizations (CROs).
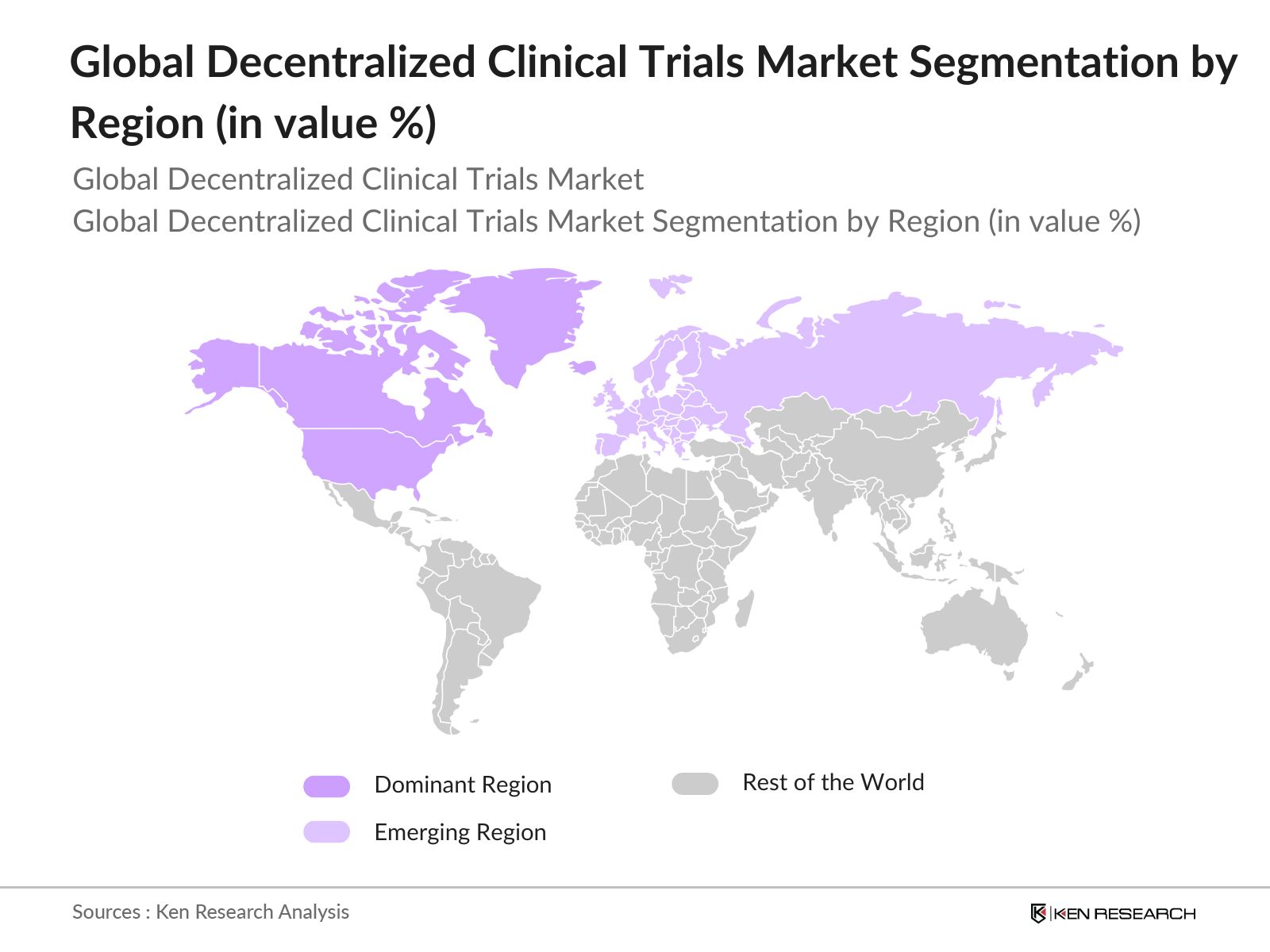
- North America, particularly the United States, leads the global DCT market due to its advanced healthcare infrastructure, extensive use of digital technology, and strong support from regulatory bodies such as the FDA. European countries like Germany and the United Kingdom are also at the forefront, benefiting from regulatory support and a high concentration of CROs. In the Asia Pacific, Japan and China have shown notable growth, driven by an expanding healthcare sector and increasing investments in clinical research.
- In September 2024, the U.S. Food and Drug Administration (FDA) released comprehensive guidelines for conducting decentralized clinical trials. These guidelines outline best practices for remote data collection, patient monitoring, and the use of digital health technologies, aiming to ensure the integrity and reliability of DCTs. The FDA emphasizes the importance of maintaining data quality and patient safety, providing a framework that supports innovation while upholding rigorous scientific standards. This regulatory clarity has encouraged broader adoption of decentralized methodologies in clinical research.
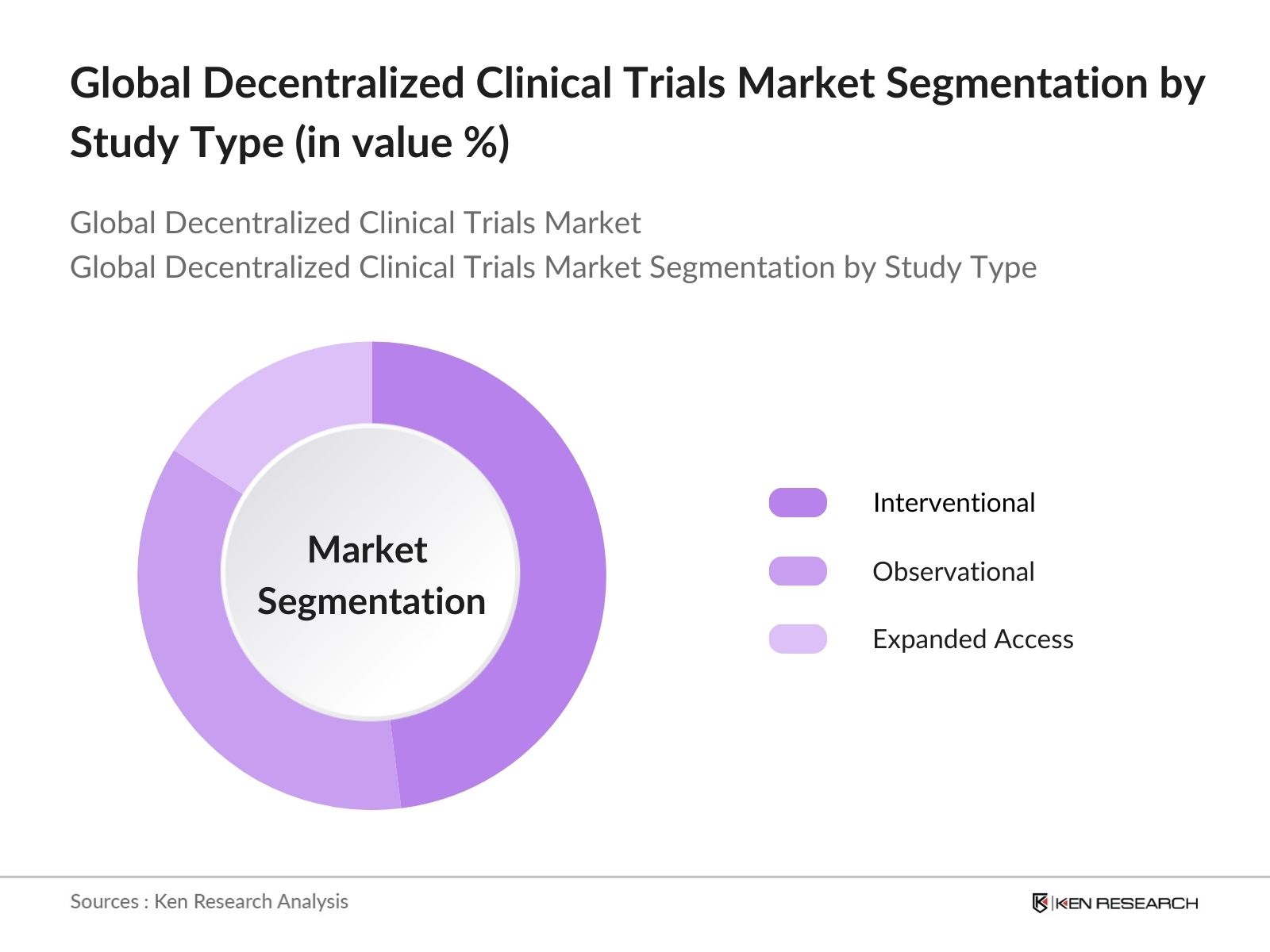
Global Decentralized Clinical Trials Market Segmentation
- By Study Type: The DCT market is segmented by study type into interventional trials, observational trials, and expanded access trials. Interventional trials dominate the market, primarily due to the high demand for testing new therapeutics under controlled conditions. These trials are essential for evaluating the efficacy and safety of novel drugs and therapies, and DCTs allow for broader geographic reach, enhancing data diversity and validity.
- By Therapeutic Area: The DCT market is categorized by therapeutic area, including oncology, cardiology, endocrinology, infectious diseases, and neurology. Oncology is the leading segment due to the critical need for innovative cancer treatments and the complexity of managing traditional cancer trials. DCTs in oncology facilitate remote patient monitoring and data collection, making trials more accessible for patients and enabling real-time data analysis.
- By Region: The market is divided into North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America holds the largest share due to the advanced healthcare ecosystem and extensive adoption of DCTs by major pharma companies and CROs. Europe follows closely, with favorable regulatory policies and a robust clinical research infrastructure. Asia Pacific is witnessing rapid growth, fueled by increasing clinical trial outsourcing and supportive government initiatives in countries like China and India.
Global Decentralized Clinical Trials Market Competitive Landscape
The global DCT market is highly consolidated, with a few major players dominating the landscape. Companies like Medable Inc., Science 37, and ICON Plc lead due to their strong technological capabilities and extensive clinical trial expertise. Strategic partnerships and continuous technological innovation are key competitive factors.
Global Decentralized Clinical Trials Market Analysis
Market Growth Drivers
- Digital Health Technology Adoption: The adoption of digital health technologies has enhanced the efficiency and reach of decentralized clinical trials (DCTs). In 2024, the global number of smartphone users reached approximately 6.8 billion, facilitating widespread use of mobile health applications for remote patient monitoring and data collection. Additionally, the proliferation of wearable devices, with over 1.1 billion units in use globally, has enabled continuous health data tracking, supporting real-time monitoring in clinical research. These advancements have streamlined data acquisition and improved patient engagement in DCTs.
- Increased Access to Diverse Patient Populations: Decentralized clinical trials have expanded access to diverse patient populations by overcoming geographical and logistical barriers. In 2024, approximately 55% of the global population resided in urban areas, leaving a notable portion in rural regions. DCTs have enabled participation from these underserved areas, enhancing the representativeness of clinical studies. For instance, the inclusion of participants from various regions has increased by 20% in recent trials, leading to more comprehensive and generalizable data.
- Patient-Centric Clinical Models: The shift towards patient-centric clinical models has been a pivotal driver for DCTs. In 2024, patient retention rates in decentralized trials improved by 15% compared to traditional models, attributed to the flexibility and convenience offered. Remote participation options have reduced the burden on patients, leading to higher satisfaction and adherence. This approach aligns with the growing emphasis on patient-centered care in healthcare systems worldwide.
Market Challenges
- Data Security and Privacy Concerns: The expansion of decentralized clinical trials has heightened concerns regarding data security and patient privacy. In 2024, there were over 1,000 reported data breaches in the healthcare sector globally, exposing sensitive patient information. Ensuring compliance with regulations such as the Health Insurance Portability and Accountability Act (HIPAA) in the U.S. and the General Data Protection Regulation (GDPR) in Europe remains a major challenge for DCTs, necessitating robust cybersecurity measures and protocols.
- Operational Complexity in Multi-Site Studies: Conducting decentralized clinical trials across multiple sites introduces operational complexities, including coordinating logistics, standardizing procedures, and managing diverse regulatory requirements. In 2024, approximately 60% of DCTs involved multiple countries, each with distinct regulatory landscapes. This complexity can lead to delays and increased costs, underscoring the need for streamlined processes and effective project management strategies.
Global Decentralized Clinical Trials Market Future Outlook
The Decentralized Clinical Trials market is anticipated to continue its growth trajectory over the next five years, driven by advancements in digital health technologies, increasing demand for remote patient monitoring, and continued regulatory support. Growing investments from both public and private sectors are expected to further accelerate the adoption of DCTs across diverse therapeutic areas.
Market Opportunities
- Expansion into Emerging Markets: Emerging markets offer substantial growth potential for decentralized clinical trials. In 2024, countries in Asia and Africa accounted for 60% of the global population, yet were underrepresented in clinical research. Expanding DCTs into these regions can enhance participant diversity and provide insights into varied genetic backgrounds and disease profiles, ultimately leading to more inclusive and effective healthcare solutions.
- Collaborations with Digital Health Companies: Collaborations between clinical trial sponsors and digital health companies can drive innovation and efficiency in decentralized trials. In 2024, there were over 2,000 digital health startups globally, offering a range of technologies from telemedicine platforms to data analytics tools. Partnering with these companies can provide access to cutting-edge solutions, streamline trial processes, and enhance patient engagement through user-friendly interfaces and personalized communication channels.
Scope of the Report
|
Study Type |
Interventional Trials Observational Trials Expanded Access Trials |
|
Therapeutic Area |
Oncology Cardiology Endocrinology Infectious Diseases Neurology |
|
Component |
Wearable Devices eConsent Solutions Telemedicine Platforms Data Management Software |
|
End-User |
Pharmaceutical Companies CROs Biotech Companies Hospitals and Research Institutes |
|
Region |
North America Europe Asia Pacific Latin America Middle East & Africa |
Products
Key Target Audience
Biopharmaceutical Companies
Contract Research Organizations (CROs)
Digital Health and Technology Solution Providers
Government and Regulatory Bodies (FDA, EMA, PMDA)
Clinical Research Institutes
Venture Capitalist Firms and Investors
Medical Device Manufacturers
Data Management and Analytics Firms
Companies
Players Mentioned in the Report
Medable Inc.
Science 37
Parexel International Corporation
ICON Plc
Covance Inc.
PRA Health Sciences
Clinical Ink
Oracle Health Sciences
Medidata Solutions
Labcorp Drug Development
Table of Contents
1. Global Decentralized Clinical Trials Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy
1.3 Market Dynamics Overview
1.4 Key Market Segmentation Overview
2. Global Decentralized Clinical Trials Market Size (in USD Billion)
2.1 Historical Market Size
2.2 Year-On-Year Growth Analysis
2.3 Key Milestones and Market Developments
3. Global Decentralized Clinical Trials Market Analysis
3.1 Growth Drivers
3.1.1 Digital Health Technology Adoption
3.1.2 Increased Access to Diverse Patient Populations
3.1.3 Patient-Centric Clinical Models
3.1.4 Regulatory Support for DCTs
3.2 Market Challenges
3.2.1 Data Security and Privacy Concerns
3.2.2 Operational Complexity in Multi-Site Studies
3.2.3 Technological Infrastructure Constraints
3.2.4 Patient Compliance and Engagement
3.3 Opportunities
3.3.1 Technological Innovations in Remote Monitoring
3.3.2 Expansion into Emerging Markets
3.3.3 Collaborations with Digital Health Companies
3.3.4 Integration with AI and Big Data
3.4 Trends
3.4.1 Use of Wearable Devices for Data Collection
3.4.2 Decentralized Trials for Rare Diseases
3.4.3 Increased Use of Virtual Visits
3.4.4 Rise in Adaptive Trial Designs
3.5 Regulatory Environment
3.5.1 FDA Guidelines for Decentralized Trials
3.5.2 EU MDR and Decentralized Trials
3.5.3 HIPAA Compliance
3.5.4 National and International Collaboration Standards
3.6 SWOT Analysis
3.7 Stakeholder Ecosystem
3.8 Porters Five Forces Analysis
3.9 Competitive Landscape Overview
4. Global Decentralized Clinical Trials Market Segmentation
4.1 By Study Type (in Value %)
4.1.1 Interventional Trials
4.1.2 Observational Trials
4.1.3 Expanded Access Trials
4.2 By Therapeutic Area (in Value %)
4.2.1 Oncology
4.2.2 Cardiology
4.2.3 Endocrinology
4.2.4 Infectious Diseases
4.2.5 Neurology
4.3 By Component (in Value %)
4.3.1 Wearable Devices
4.3.2 eConsent Solutions
4.3.3 Telemedicine Platforms
4.3.4 Data Management Software
4.4 By End-User (in Value %)
4.4.1 Pharmaceutical Companies
4.4.2 CROs
4.4.3 Biotech Companies
4.4.4 Hospitals and Research Institutes
4.5 By Region (in Value %)
4.5.1 North America
4.5.2 Europe
4.5.3 Asia Pacific
4.5.4 Latin America
4.5.5 Middle East & Africa
5. Global Decentralized Clinical Trials Market Competitive Analysis
5.1 Detailed Profiles of Major Competitors
5.1.1 Medable Inc.
5.1.2 Science 37
5.1.3 Parexel International Corporation
5.1.4 ICON Plc
5.1.5 Covance Inc.
5.1.6 PRA Health Sciences
5.1.7 Clinical Ink
5.1.8 Oracle Health Sciences
5.1.9 Medidata Solutions
5.1.10 Labcorp Drug Development
5.1.11 Syneos Health
5.1.12 IQVIA
5.1.13 Verily Life Sciences
5.1.14 Clario
5.1.15 Signant Health
5.2 Cross Comparison Parameters (Revenue, Headquarters, Year Established, Market Penetration, Technology Offerings, Service Portfolio, Regional Presence, Key Partnerships)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers and Acquisitions
5.6 Investment Analysis
5.7 Government and Private Funding
5.8 Strategic Collaborations and Partnerships
6. Global Decentralized Clinical Trials Market Regulatory Framework
6.1 Regulatory Standards and Guidelines
6.2 Compliance and Certification Requirements
6.3 Data Security and Privacy Laws
6.4 Ethical and Legal Considerations
7. Global Decentralized Clinical Trials Future Market Size (in USD Billion)
7.1 Projected Market Size Analysis
7.2 Key Growth Catalysts for Future Market Expansion
8. Global Decentralized Clinical Trials Future Market Segmentation
8.1 By Study Type (in Value %)
8.2 By Therapeutic Area (in Value %)
8.3 By Component (in Value %)
8.4 By End-User (in Value %)
8.5 By Region (in Value %)
9. Global Decentralized Clinical Trials Market Analysts Recommendations
9.1 Customer Analysis and Segmentation Insights
9.2 Marketing and Outreach Strategy
9.3 White Space Opportunity Analysis
9.4 Risk Mitigation Recommendations
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
In this step, a comprehensive ecosystem analysis was conducted to understand all stakeholders in the global DCT market. Extensive desk research was utilized to identify critical variables influencing market dynamics, including regulatory impacts, technological advancements, and industry collaborations.
Step 2: Market Analysis and Construction
This stage involved gathering and analyzing historical market data to assess market growth and segmentation trends. Specific attention was given to revenue generation patterns and service adoption rates across key regions.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses were formulated and validated through expert interviews, providing valuable insights from industry professionals. These consultations helped refine the understanding of market challenges and opportunities.
Step 4: Research Synthesis and Final Output
In the final phase, comprehensive data analysis was conducted to create an accurate and validated report. This involved direct engagement with clinical trial companies to obtain precise insights into market segmentation, service adoption, and key growth drivers.
Frequently Asked Questions
01. How big is the Global Decentralized Clinical Trials Market?
The global Decentralized Clinical Trials market is valued at USD 8.1 billion, driven by advancements in digital health technology and increasing demand for patient-centric trials.
02. What are the main challenges in the Global Decentralized Clinical Trials Market?
Key challenges include data security and privacy concerns, operational complexity in managing multi-site studies, and ensuring patient compliance and engagement in remote settings.
03. Who are the major players in the Global Decentralized Clinical Trials Market?
The major players include Medable Inc., Science 37, ICON Plc, Parexel, and Covance Inc. These companies lead due to their extensive technological capabilities and strong clinical trial expertise.
04. What are the growth drivers for the Global Decentralized Clinical Trials Market?
Growth is driven by the adoption of digital health technology, the need for patient-centric trials, and regulatory support from agencies like the FDA, which facilitates remote trial adoption.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















