
Global Medical Writing Market Outlook to 2030
Region:Global
Author(s):Sanjna Verma
Product Code:KROD8321
November 2024
98
About the Report
Global Medical Writing Market Overview
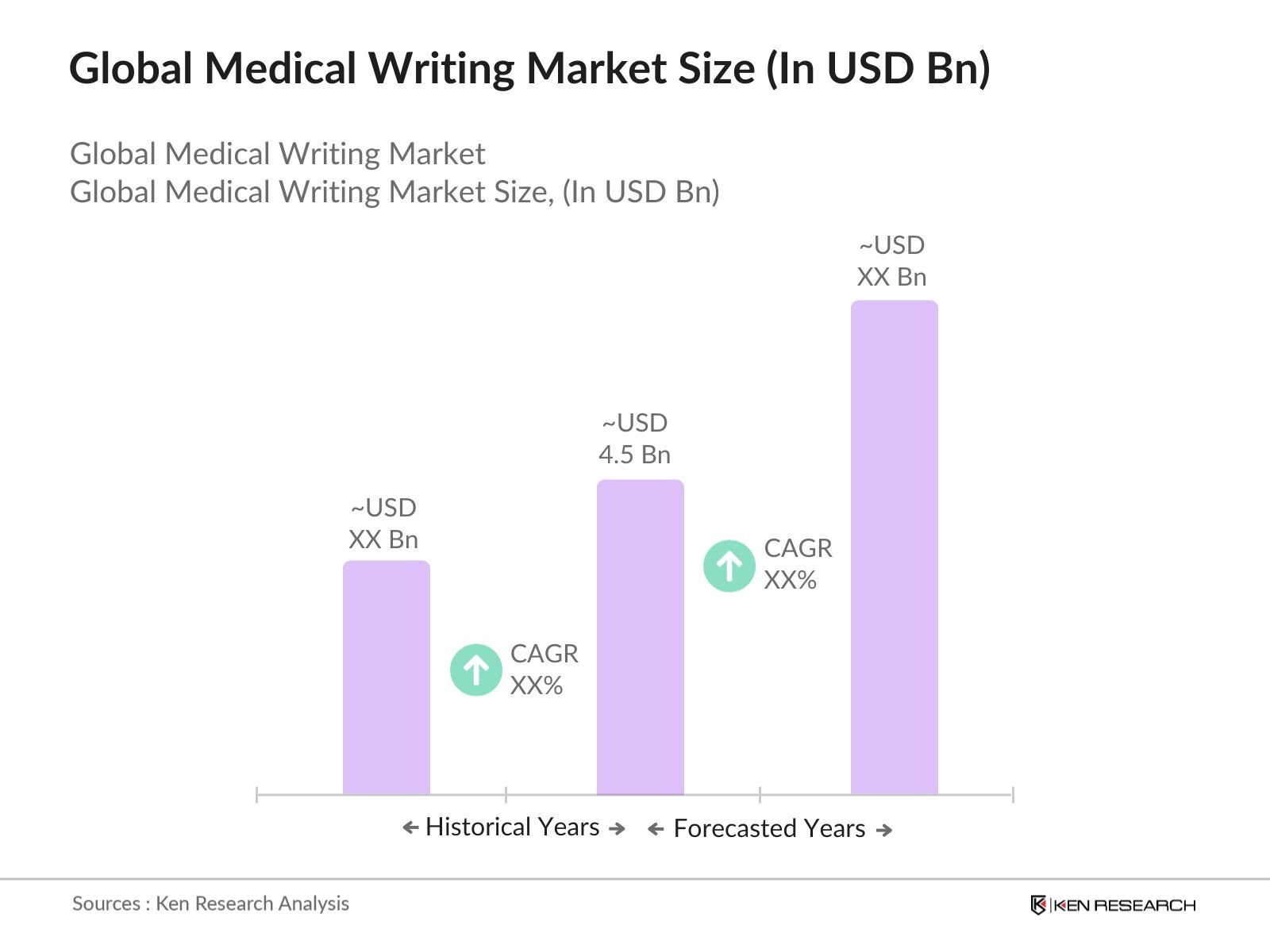
- The global medical writing market is valued at USD 4.5 billion, based on a five-year historical analysis. The markets growth is driven by the increasing complexity of clinical trials and regulatory requirements, particularly in the biopharmaceutical and pharmaceutical sectors. Key drivers include rising demand for precise documentation in clinical trials, advancements in drug development, and increased regulatory scrutiny. With a significant number of new drug approvals and heightened demand for real-world evidence reports, the medical writing market is gaining traction globally.
- Countries like the United States, Germany, and Japan dominate the medical writing market due to their advanced healthcare infrastructure and concentration of pharmaceutical and biopharmaceutical companies. The United States, in particular, is home to many top Contract Research Organizations (CROs) and global regulatory bodies, which significantly boost the demand for regulatory and clinical medical writing services.
- The U.S. Food and Drug Administration (FDA) plays a crucial role in setting standards for medical writing in regulatory submissions. In 2024, the FDA updated its guidance on the format and content of clinical trial documents, including Clinical Study Reports (CSRs) and Investigators Brochures (IBs). The guidance emphasizes clarity, accuracy, and transparency in medical documentation to facilitate regulatory review. Medical writers must now adhere to specific templates and formats when preparing submissions, ensuring that the documents are consistent and compliant with FDA regulations.
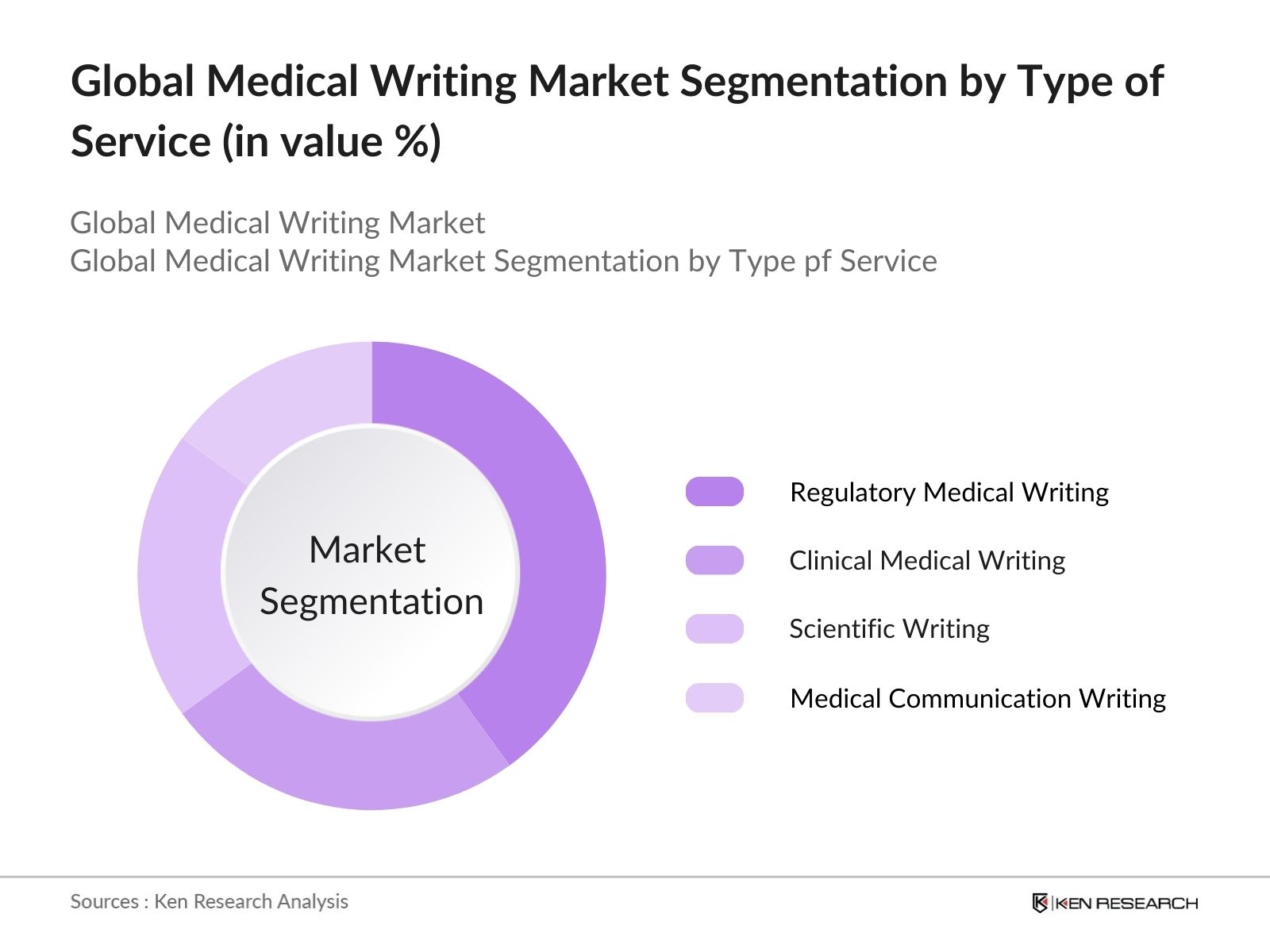
Global Medical Writing Market Segmentation
By Type of Service: The global medical writing market is segmented by type of service into regulatory medical writing, clinical medical writing, scientific writing, and medical communication writing. Recently, regulatory medical writing has held a dominant share due to the increasing need for stringent documentation in regulatory submissions to comply with global standards. Countries like the U.S. and regions such as the European Union require complex documentation that adheres to their regulatory frameworks, making regulatory medical writing an essential service for pharmaceutical and biotech companies.
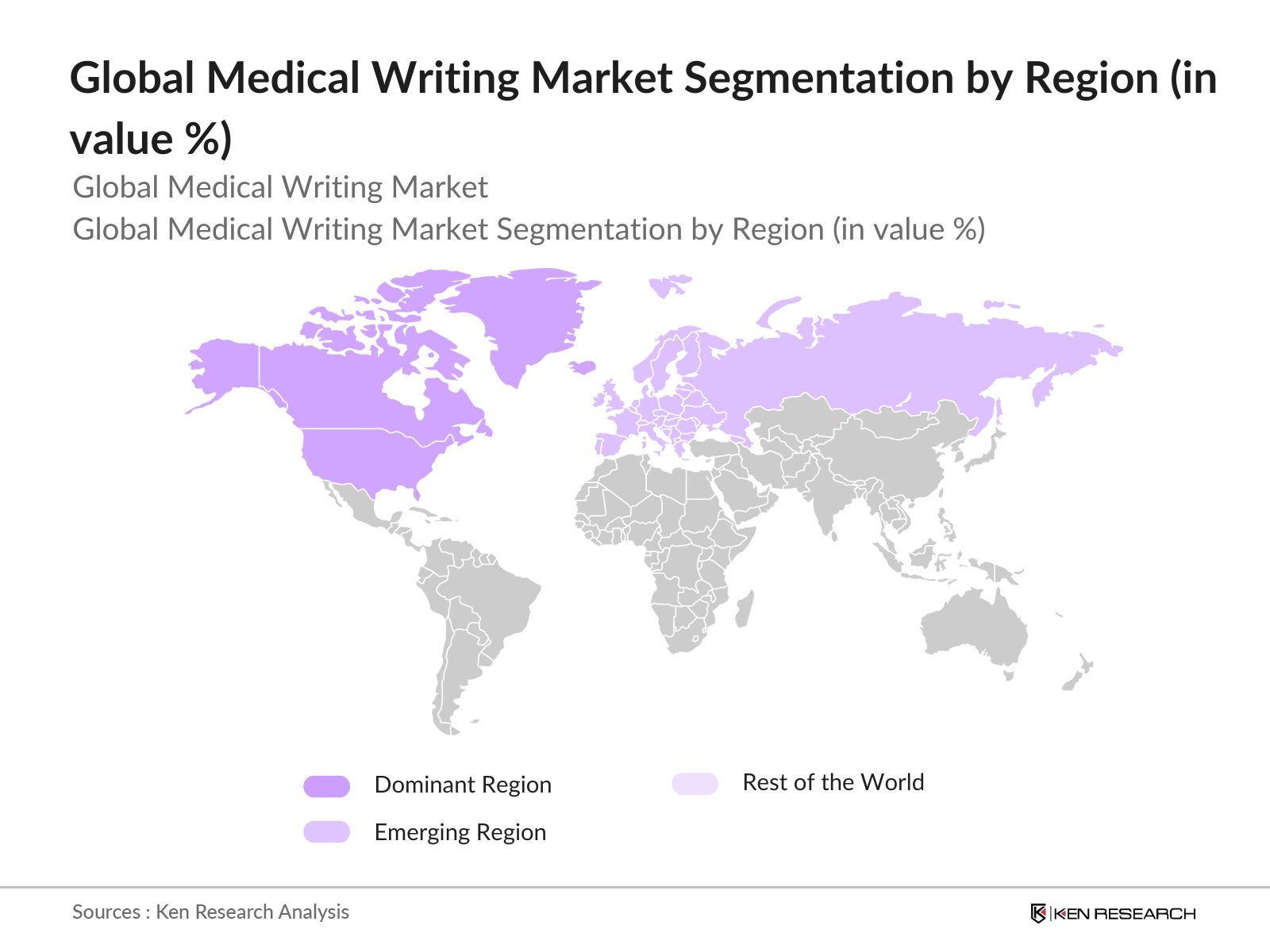
By Region: The global medical writing market is segmented by region into North America, Europe, Asia-Pacific, Latin America, and the Middle East & Africa. North America dominates the global market, primarily due to the extensive research and development activities carried out by pharmaceutical companies and the significant number of clinical trials conducted in the region. Additionally, regulatory frameworks in the U.S., such as those enforced by the FDA, demand detailed and high-quality documentation, further driving the dominance of North America in this market.
By End-User: By end-user, the market is segmented into Contract Research Organizations (CROs), pharmaceutical companies, biotechnology firms, and academic institutions. CROs have captured the largest market share due to their prominent role in outsourcing medical writing tasks. The rising outsourcing trends, particularly in clinical and regulatory writing, have propelled CROs as the largest consumers of medical writing services, especially as they handle the growing number of clinical trials worldwide.
Global Medical Writing Market Competitive Landscape
The global medical writing market is dominated by a mix of large, well-established players and smaller, specialized agencies. The consolidation of key players highlights their influence and dominance across regulatory, clinical, and scientific writing services.
|
Company Name |
Establishment Year |
Headquarters |
Number of Employees |
Specialization |
Regulatory Expertise |
Global Presence |
Partnerships with CROs |
Technological Adoption |
R&D Focus |
|
Parexel International |
1982 |
Boston, USA |
- |
- |
- |
- |
- |
- |
- |
|
IQVIA |
1982 |
Durham, USA |
- |
- |
- |
- |
- |
- |
- |
|
ICON plc |
1990 |
Dublin, Ireland |
- |
- |
- |
- |
- |
- |
- |
|
Medpace |
1992 |
Cincinnati, USA |
- |
- |
- |
- |
- |
- |
- |
|
Envision Pharma Group |
2001 |
Horsham, UK |
- |
- |
- |
- |
- |
- |
- |
Global Medical Writing Market Analysis
Growth Drivers
- Increasing Clinical Trial Volumes: The global medical writing market is witnessing significant growth due to the increasing volume of clinical trials worldwide. According to the World Health Organization (WHO), over 375,000 clinical trials were registered globally by 2024, marking an increase from 201,000 trials in 2018. The surge in clinical trials has created a heightened demand for medical writing services, especially in documentation related to regulatory submissions and clinical study reports. This increase is driven by the growing development of vaccines, novel therapeutics, and biologics.
- Rising Regulatory Approvals for New Therapies: With the rise in new drug and therapy approvals by regulatory authorities, the demand for regulatory medical writing has surged. The U.S. Food and Drug Administration (FDA) approved 59 novel drugs in 2023, an increase from 50 approvals in 2022. This rise in approvals requires extensive documentation, including Investigational New Drug (IND) applications, New Drug Applications (NDAs), and Biologics License Applications (BLAs), necessitating highly specialized medical writing skills, which contributed to the growing need for professional medical writers skilled in regulatory documentation.
- Adoption of Artificial Intelligence in Medical Writing: AI tools can automatically generate clinical trial reports, regulatory documents, and safety summaries by analyzing vast amounts of trial data. By 2024, AI-powered medical writing tools were implemented by over 50% of leading biopharmaceutical companies, significantly reducing turnaround times for regulatory submissions. This shift has increased the demand for medical writers who can work alongside AI technologies to enhance the quality of medical documentation. The AI healthcare market reached $11 billion in 2024, influencing medical writing practices.
Challenges
- Complex Regulatory Requirements: The medical writing industry faces significant challenges in navigating complex global and regional regulatory requirements. Regulatory bodies like the FDA, EMA, and Japan's Pharmaceuticals and Medical Devices Agency (PMDA) each have unique requirements for documentation formats, terminology, and clinical data reporting. For instance, in 2023, the FDA revised its clinical trial reporting guidelines, mandating more detailed trial data submissions for both safety and efficacy outcomes.
- High Competition Among CROs and Freelancers: The medical writing sector faces intense competition from Contract Research Organizations (CROs) and freelancers. In 2024, more than 25,000 freelance medical writers were offering their services globally, making it challenging for CROs and full-time writers to maintain competitive pricing. Additionally, the growing demand for specialized documents such as risk management plans and pharmacovigilance reports has led to increased competition. This competition often results in cost pressure, forcing organizations to reduce their fees, affecting the overall profitability of the medical writing market.
Global Medical Writing Market Future Outlook
Global Medical Writing Market is expected to experience significant growth, driven by the rising demand for clinical trial documentation, regulatory submissions, and real-world evidence reports. The continuous evolution of artificial intelligence and automation in medical writing will enhance productivity, while increasing drug development activities and regulatory approvals will create more opportunities for specialized medical writing services. Companies will likely increase investments in skilled professionals and technology to meet the rising complexity of documentation requirements.
Market Opportunities
- Growing Demand for Real-World Evidence (RWE) Documents: The increasing emphasis on Real-World Evidence (RWE) in clinical trials and post-marketing surveillance presents a lucrative opportunity for medical writing services. Regulatory agencies like the FDA have been integrating RWE into their decision-making processes, with over 100 submissions incorporating RWE in 2023. Medical writers are now tasked with producing high-quality RWE documents, which require the synthesis of large volumes of observational data and real-world patient outcomes.
- Market Expansion into Emerging Regions: The medical writing market is experiencing expansion in emerging regions, including Asia-Pacific and Latin America, due to the rise in clinical trials and biopharmaceutical research. For instance, India alone conducted over 3,000 clinical trials in 2023, while Brazil saw a 15% increase in biopharmaceutical research. This expansion has increased the demand for region-specific medical writing services, particularly for regulatory documentation and clinical study reports. As more biopharma companies establish research centers in these regions, the market for medical writing will continue to grow.
Scope of the Report
|
Segment |
Sub-Segments |
|
By Type of Service |
Regulatory Medical Writing Clinical Medical Writing Scientific Writing Medical Communication Writing |
|
By End-User |
Contract Research Organizations (CROs Pharmaceutical Companies Biotechnology Firms Academic and Research Institutions |
|
By Therapeutic Area |
Oncology Cardiology Neurology Rare Diseases |
|
By Document Type |
Clinical Study Reports (CSR) Investigators Brochures (IB) Patient Information Leaflets (PIL) Drug Safety Updates |
|
By Region |
North America Europe Asia-Pacific Latin America Middle East & Africa |
Products
Key Target Audience
Contract Research Organizations (CROs)
Pharmaceutical Companies
Biotechnology Firms
Academic and Research Institutions
Government and Regulatory Bodies (FDA, EMA, PMDA)
Medical Writing Agencies
Investors and Venture Capitalist Firms
Pharmaceutical R&D Departments
Companies
Players Mentioned in the Report
Parexel International
IQVIA
ICON plc
Medpace
Syneos Health
Freyr Solutions
Envision Pharma Group
Trilogy Writing & Consulting
Proscribe Medical Writing
Covance (Labcorp Drug Development)
Table of Contents
1. Global Medical Writing Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (Medical Writing Output Volume)
1.4. Market Segmentation Overview (Clinical, Regulatory, Scientific, Medical Communications)
2. Global Medical Writing Market Size (In USD Bn)
2.1. Historical Market Size (In-Depth Review of Clinical and Regulatory Writing)
2.2. Year-On-Year Growth Analysis (CRO Adoption, Drug Approval Rates)
2.3. Key Market Developments and Milestones (Notable Industry Mergers, Acquisitions, Partnerships)
3. Global Medical Writing Market Analysis
3.1. Growth Drivers
3.1.1. Increasing Clinical Trial Volumes
3.1.2. Rising Regulatory Approvals for New Therapies
3.1.3. Adoption of Artificial Intelligence in Medical Writing
3.1.4. Expansion of Biopharmaceutical Companies
3.2. Market Challenges
3.2.1. Complex Regulatory Requirements (Global and Regional)
3.2.2. High Competition Among CROs and Freelancers
3.2.3. Talent Shortages in Regulatory Medical Writing
3.3. Opportunities
3.3.1. Growing Demand for Real-World Evidence (RWE) Documents
3.3.2. Market Expansion into Emerging Regions
3.3.3. Digital Transformation in Medical Documentation
3.3.4. Collaboration with Academic Institutions for Scientific Writing
3.4. Trends
3.4.1. Integration of Cloud-Based Documentation Platforms
3.4.2. Demand for Plain Language Summaries (PLS)
3.4.3. Outsourcing to Specialized Medical Writing Agencies
3.4.4. Use of Automation Tools for Regulatory Submissions
3.5. Government Regulation
3.5.1. FDA Guidance on Medical Writing Standards
3.5.2. EMA Regulatory Framework for Medical Writing
3.5.3. ICH Guidelines for Clinical Documentation
3.5.4. Data Transparency Laws and Reporting Requirements
3.6. SWOT Analysis (Strengths, Weaknesses, Opportunities, Threats)
3.7. Stake Ecosystem (Key Market Stakeholders, End-Users)
3.8. Porters Five Forces (Supplier Power, Buyer Power, Substitution Threats, etc.)
3.9. Competition Ecosystem (Top Global Players, Niche Players)
4. Global Medical Writing Market Segmentation
4.1. By Type of Service (In Value %)
4.1.1. Regulatory Medical Writing
4.1.2. Clinical Medical Writing
4.1.3. Scientific Writing
4.1.4. Medical Communication Writing
4.2. By End-User (In Value %)
4.2.1. Contract Research Organizations (CROs)
4.2.2. Pharmaceutical Companies
4.2.3. Biotechnology Firms
4.2.4. Academic and Research Institutions
4.3. By Therapeutic Area (In Value %)
4.3.1. Oncology
4.3.2. Cardiology
4.3.3. Neurology
4.3.4. Rare Diseases
4.4. By Document Type (In Value %)
4.4.1. Clinical Study Reports (CSR)
4.4.2. Investigators Brochures (IB)
4.4.3. Patient Information Leaflets (PIL)
4.4.4. Drug Safety Updates
4.5. By Region (In Value %)
4.5.1. North America
4.5.2. Europe
4.5.3. Asia-Pacific
4.5.4. Latin America
4.5.5. Middle East & Africa
5. Global Medical Writing Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Parexel International
5.1.2. IQVIA
5.1.3. Medpace
5.1.4. ICON plc
5.1.5. Covance (Labcorp Drug Development)
5.1.6. Syneos Health
5.1.7. Freyr Solutions
5.1.8. Envision Pharma Group
5.1.9. Trilogy Writing & Consulting
5.1.10. Proscribe Medical Writing
5.2. Cross Comparison Parameters (No. of Employees, Regulatory Expertise, Therapeutic Focus, Regions Served, M&A Activities, Technological Adoption, Strategic Partnerships, Financial Strength)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers And Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. Global Medical Writing Market Regulatory Framework
6.1. International Guidelines (ICH, WHO)
6.2. Regional Regulations (FDA, EMA, PMDA)
6.3. Compliance Requirements (Good Clinical Practice Guidelines, Data Protection)
6.4. Certification Processes (ISO, HIPAA, GDPR)
7. Global Medical Writing Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth (Digital Transformation, Real-World Data Adoption)
8. Global Medical Writing Future Market Segmentation
8.1. By Type of Service (In Value %)
8.2. By End-User (In Value %)
8.3. By Therapeutic Area (In Value %)
8.4. By Document Type (In Value %)
8.5. By Region (In Value %)
9. Global Medical Writing Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves identifying the essential factors influencing the global medical writing market. This includes mapping key stakeholders, understanding regulatory requirements, and examining demand drivers in pharmaceutical and clinical documentation. Extensive desk research is carried out using secondary databases and proprietary sources to pinpoint critical variables impacting the market.
Step 2: Market Analysis and Construction
In this phase, historical data related to medical writing services is compiled and analyzed. This involves evaluating the number of clinical trials, the growth of biopharmaceutical companies, and the global spread of Contract Research Organizations (CROs). Key market developments and service quality statistics are used to ensure reliable data estimates.
Step 3: Hypothesis Validation and Expert Consultation
Hypotheses regarding the market are tested through interviews with industry experts and professionals from the medical writing field. Computer-assisted telephone interviews (CATIs) and expert consultation provide operational and financial insights from key market players. This process helps validate market data and trends.
Step 4: Research Synthesis and Final Output
The final step includes synthesizing the gathered data, incorporating insights from multiple players in the industry, including pharmaceutical companies, CROs, and medical writing agencies. This ensures that the final report presents an accurate and comprehensive analysis of the global medical writing market.
Frequently Asked Questions
1. How big is the Global Medical Writing Market?
The global medical writing market was valued at USD 4.5 billion and is primarily driven by the increasing complexity of clinical trials and rising regulatory requirements in the biopharmaceutical and pharmaceutical sectors.
2. What are the challenges in the Global Medical Writing Market?
Challenges in global medical writing market include high competition among medical writing agencies and freelancers, complex regulatory frameworks across different countries, and the shortage of skilled professionals capable of handling complex regulatory submissions.
3. Who are the major players in the Global Medical Writing Market?
Major players in global medical writing market include Parexel International, IQVIA, ICON plc, Medpace, and Syneos Health. These companies have established strong global presences and partnerships with pharmaceutical companies and CROs.
4. What are the growth drivers of the Global Medical Writing Market?
The global medical writing market is propelled by the increasing number of clinical trials, stringent regulatory requirements, rising demand for real-world evidence documentation, and advancements in drug development activities across the globe.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















