
Global Pharmaceutical Manufacturing Market Outlook to 2030
Region:Global
Author(s):Yogita Sahu
Product Code:KROD7442
November 2024
83
About the Report
Global Pharmaceutical Manufacturing Market Overview
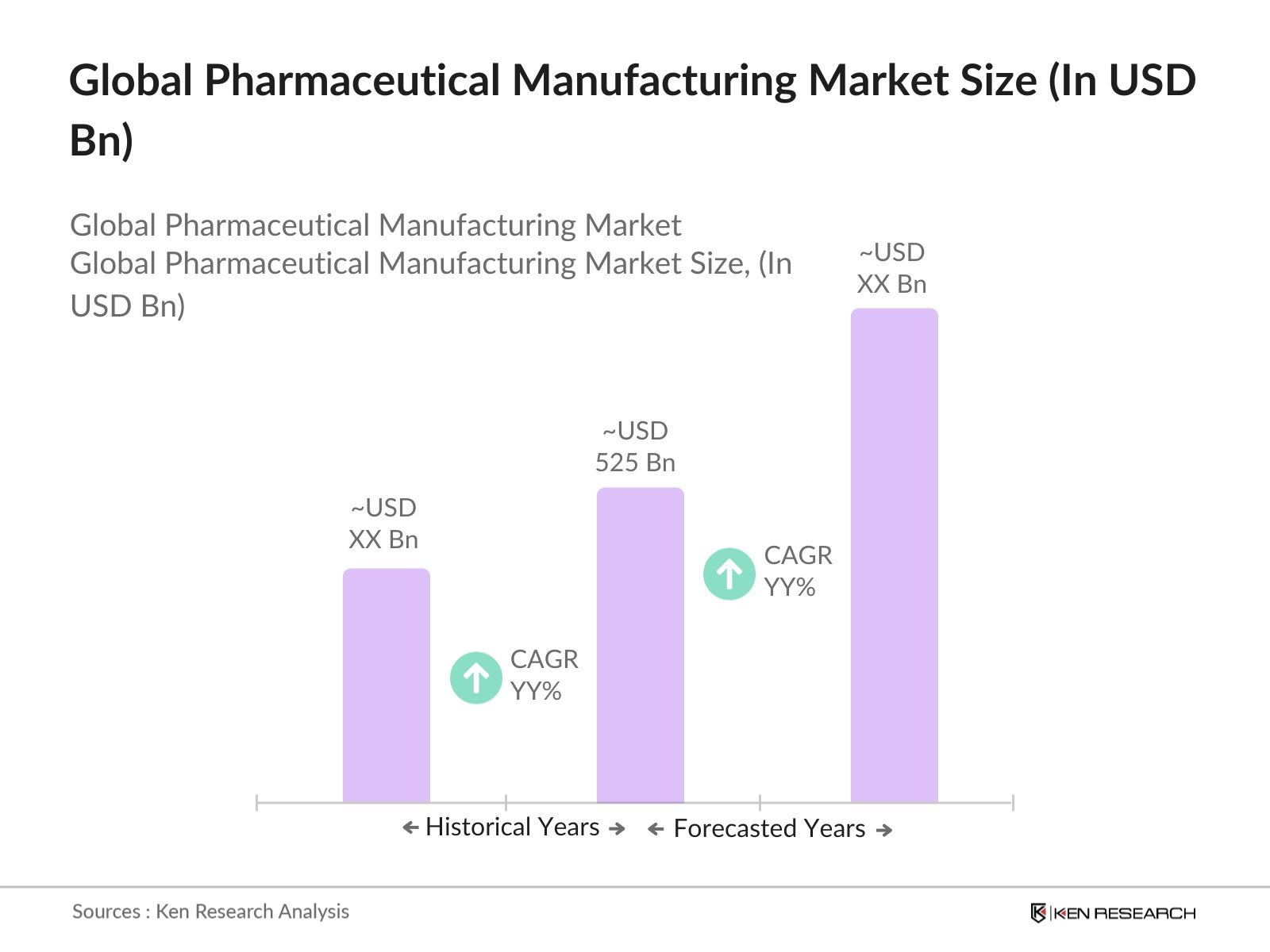
- The global pharmaceutical manufacturing market, valued at USD 525 billion, is propelled by increasing investment in research and development, regulatory approval of innovative therapies, and rising demand for both generic and specialty drugs. This growth is sustained by a continuous influx of advanced manufacturing technologies, such as single-use systems and continuous manufacturing processes.
- The pharmaceutical manufacturing sector is dominated by key regions, including the United States, Europe, and China, primarily due to their established regulatory frameworks, robust research facilities, and availability of skilled workforce. The U.S. benefits from its extensive investment in biotechnology and high expenditure on healthcare, while Europe and China leverage government support and growing patient populations to sustain their leading positions.
- Governments globally have allocated approximately $20 billion in incentives to promote local pharmaceutical manufacturing. These initiatives are aimed at reducing dependency on imports and enhancing domestic production capabilities, especially for essential medications and critical APIs, boosting regional manufacturing sectors and creating a more resilient pharmaceutical supply chain.
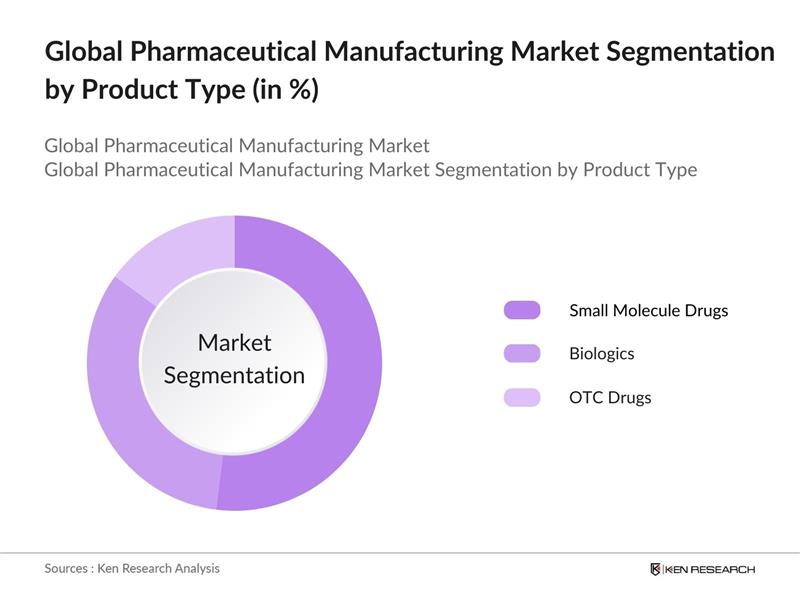
Global Pharmaceutical Manufacturing Market Segmentation
By Product Type: The market is segmented by product type into small molecule drugs, biologics, and over-the-counter (OTC) drugs. Small molecule drugs dominate the segment due to their simpler production process, extensive applications across multiple therapeutic areas, and established role in generic drug manufacturing. Biologics, while requiring more complex manufacturing processes, are also growing due to their efficacy in treating chronic and rare diseases. OTC drugs remain crucial as consumer demand for accessible healthcare products continues to rise.
By Application: The market is categorized by application into oncology, cardiovascular, neurology, and infectious diseases. Oncology holds a significant share due to a higher prevalence of cancer cases globally and continuous innovation in targeted therapies. Neurology and cardiovascular segments are also prominent, driven by aging populations and increasing incidences of neurological and cardiovascular conditions, respectively. Infectious diseases remain vital due to ongoing demand for antibiotics and antiviral drugs.
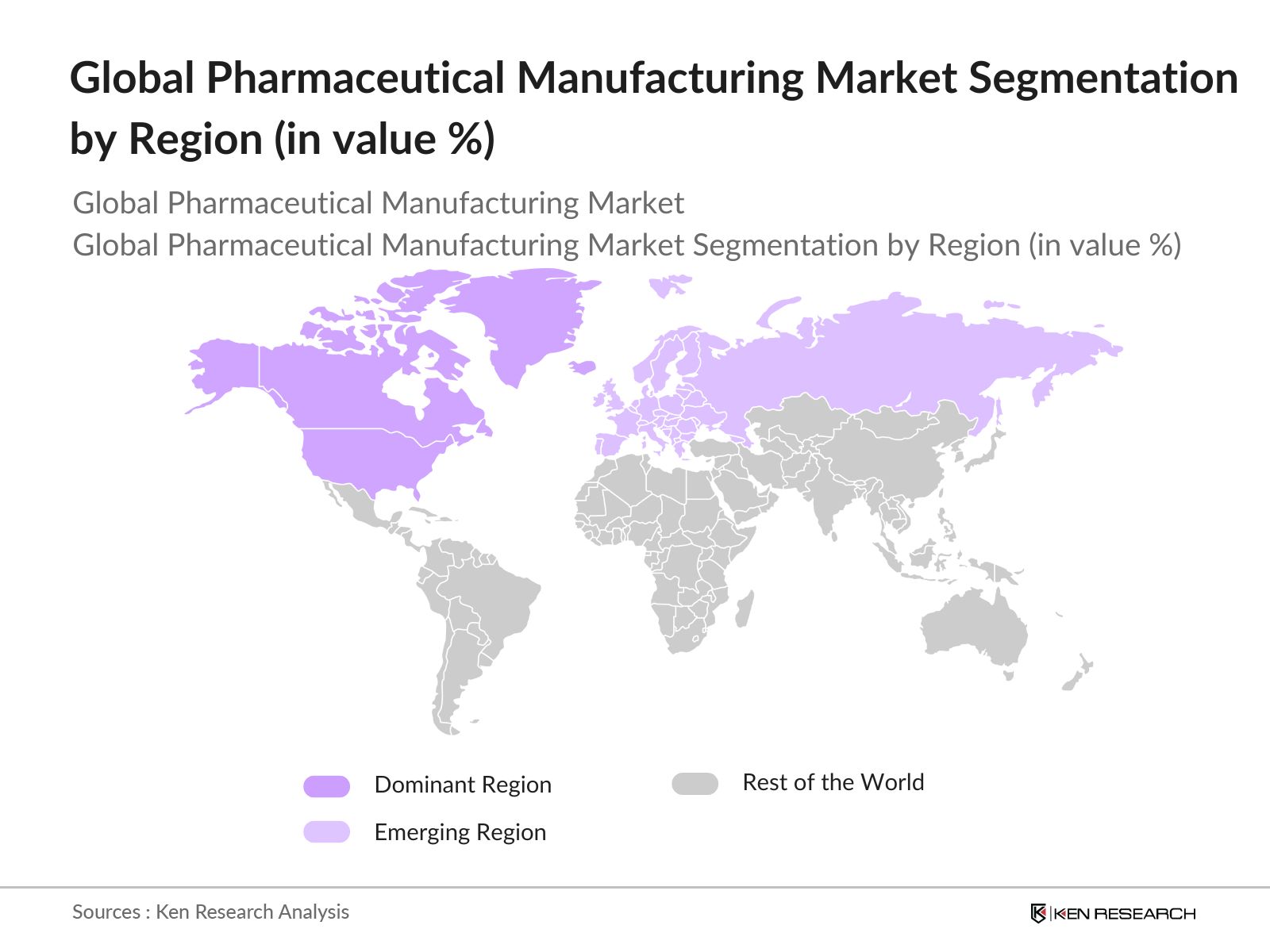
By Region: The market is segmented regionally into North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa. North America dominates due to its well-established healthcare infrastructure and substantial pharmaceutical R&D investment. Asia-Pacific follows closely, benefitting from lower manufacturing costs and increasing demand for pharmaceuticals, while Europe leverages strong government support for pharmaceutical innovation. The market in Latin America and Middle East & Africa is expanding as healthcare access improves and demand for affordable drugs rises.
Global Pharmaceutical Manufacturing Market Competitive Landscape
The market is concentrated among key players who drive innovation and maintain significant influence over global supply chains.
Global Pharmaceutical Manufacturing Market Analysis
Market Growth Drivers
- Rising Demand for Personalized Medicine: The market is experiencing increased demand for personalized medicine, fueled by a growing aging population and chronic disease prevalence. Globally, around 703 million people are aged 65 or older, and this demographic is projected to rise to nearly 1.5 billion by 2050. This increase necessitates advanced, individualized treatments to address age-specific health issues, boosting the demand for personalized medicine production.
- Expansion of Biopharmaceuticals: Biopharmaceuticals, such as monoclonal antibodies, are becoming central in pharmaceutical manufacturing, with over 500 drugs currently in development globally. Biopharmaceutical facilities now encompass around 30% of the overall pharmaceutical production infrastructure, reflecting the markets shift towards biologics. This shift is driven by the high demand for more effective and targeted treatments, particularly in oncology, with 16 million new cancer cases recorded annually.
- Global Vaccine Production Boosts Capacity: The demand for vaccine production has surged, especially post-COVID-19, with global vaccine doses reaching over 12 billion in the last two years. To meet rising needs, pharmaceutical manufacturers are heavily investing in new facilities and partnerships to boost manufacturing capacity.
Market Challenges
- Supply Chain Disruptions in Critical Raw Materials: The industry faces ongoing supply chain challenges for active pharmaceutical ingredients (APIs), with raw material shortages costing the industry over $3 billion in additional procurement and logistical costs in the past two years. Key ingredients sourced from limited suppliers are vulnerable to geopolitical tensions and transportation disruptions, leading to production delays and increased costs in securing alternative sources.
- Shortage of Skilled Labor: There is a global shortage of skilled labor in pharmaceutical manufacturing, with the industry requiring around 120,000 additional skilled workers in research and production over the next five years. This talent gap affects the timely production and quality assurance of pharmaceuticals, with training and recruitment adding an estimated $15 billion to industry costs annually.
Global Pharmaceutical Manufacturing Market Future Outlook
The global pharmaceutical manufacturing industry is expected to witness growth, driven by increasing demand for biologics, innovations in personalized medicine, and continued R&D investment.
Future Market Opportunities
- Expansion of Modular Biomanufacturing Facilities: The market will likely see a surge in modular biomanufacturing facilities, which can be rapidly assembled and customized for specific drugs, including biologics and gene therapies. These modular setups are anticipated to reduce setup costs by 30% and expedite production timelines, allowing for swift response to demand changes, particularly in personalized medicine.
- Adoption of Sustainable Pharmaceutical Practices: By 2028, it is projected that 70% of pharmaceutical manufacturers will implement sustainable practices, such as waste reduction technologies and renewable energy sources. The shift is driven by global pressure to reduce carbon footprints, with major economies enacting regulations mandating sustainable practices in pharmaceutical production.
Scope of the Report
|
Product Type |
Small Molecule Drugs |
|
Application |
Oncology |
|
Manufacturing Process |
API Manufacturing |
|
Technology |
Bioprocessing Technology |
|
Region |
North America |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Pharmaceutical Manufacturing Companies
Biotechnology Firms
API Manufacturers
Hospital and Healthcare Institutions
Research and Development Centers
Government and Regulatory Bodies (FDA, EMA, WHO)
Investor and Venture Capitalist Firms
Companies
Pfizer Inc.
Novartis AG
Johnson & Johnson
Roche Holding AG
Merck & Co., Inc.
GlaxoSmithKline plc
Sanofi S.A.
AbbVie Inc.
Eli Lilly and Company
Amgen Inc.
Table of Contents
1. Global Pharmaceutical Manufacturing Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Manufacturing Process Overview
1.4. Market Growth Rate
2. Global Pharmaceutical Manufacturing Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-on-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. Global Pharmaceutical Manufacturing Market Analysis
3.1. Growth Drivers
3.1.1. R&D Expenditure
3.1.2. Technological Advancements (e.g., Biologics Manufacturing, AI in Drug Production)
3.1.3. Regulatory Support
3.1.4. Expansion of Contract Manufacturing Organizations (CMOs)
3.2. Market Challenges
3.2.1. High Cost of Equipment and Infrastructure
3.2.2. Stringent Regulatory Compliance
3.2.3. Supply Chain Vulnerabilities
3.3. Opportunities
3.3.1. Expansion into Emerging Markets
3.3.2. Adoption of Personalized Medicine
3.3.3. Innovation in API Synthesis and Formulations
3.4. Trends
3.4.1. Biopharmaceutical Manufacturing
3.4.2. Automation and Robotics Integration
3.4.3. Digitalization and Industry 4.0
3.5. Government Regulations
3.5.1. FDA and EMA Guidelines
3.5.2. Good Manufacturing Practice (GMP) Compliance
3.5.3. Quality-by-Design (QbD) Framework
3.5.4. Global Harmonization of Standards
3.6. SWOT Analysis
3.7. Supply Chain Ecosystem
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. Global Pharmaceutical Manufacturing Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Small Molecule Drugs
4.1.2. Biologics
4.1.3. Over-the-Counter (OTC) Drugs
4.2. By Application (In Value %)
4.2.1. Oncology
4.2.2. Cardiovascular
4.2.3. Neurology
4.2.4. Infectious Diseases
4.3. By Manufacturing Process (In Value %)
4.3.1. Active Pharmaceutical Ingredient (API) Manufacturing
4.3.2. Drug Product Manufacturing
4.3.3. Formulation Development
4.4. By Technology (In Value %)
4.4.1. Bioprocessing Technology
4.4.2. Single-Use Manufacturing
4.4.3. Continuous Manufacturing
4.4.4. Additive Manufacturing (3D Printing)
4.5. By Region (In Value %)
4.5.1. North America
4.5.2. Europe
4.5.3. Asia-Pacific
4.5.4. Latin America
4.5.5. Middle East & Africa
5. Global Pharmaceutical Manufacturing Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1. Pfizer Inc.
5.1.2. Novartis AG
5.1.3. Johnson & Johnson
5.1.4. Merck & Co., Inc.
5.1.5. Roche Holding AG
5.1.6. Sanofi S.A.
5.1.7. GlaxoSmithKline plc
5.1.8. Bristol-Myers Squibb
5.1.9. AbbVie Inc.
5.1.10. Eli Lilly and Company
5.1.11. Amgen Inc.
5.1.12. AstraZeneca
5.1.13. Boehringer Ingelheim
5.1.14. Takeda Pharmaceutical Company
5.1.15. Bayer AG
5.2 Cross Comparison Parameters (Revenue, Global Presence, Manufacturing Facilities, R&D Investment, Patent Portfolio, Number of Products, Therapeutic Areas Covered, Regulatory Approvals)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Private Equity and Venture Capital Investments
5.8. Government Grants
6. Global Pharmaceutical Manufacturing Market Regulatory Framework
6.1. Good Manufacturing Practice (GMP) Standards
6.2. Regulatory Compliance and Certification
6.3. Certification Processes
7. Global Pharmaceutical Manufacturing Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. Global Pharmaceutical Manufacturing Future Market Segmentation
8.1. By Product Type (In Value %)
8.2. By Application (In Value %)
8.3. By Manufacturing Process (In Value %)
8.4. By Technology (In Value %)
8.5. By Region (In Value %)
9. Global Pharmaceutical Manufacturing Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
This stage begins with mapping the entire pharmaceutical manufacturing ecosystem, identifying all relevant stakeholders. We employ a blend of desk research and database sourcing to recognize influential market drivers and barriers that shape the industry.
Step 2: Market Analysis and Construction
Historical market data is collected to analyze trends and patterns within the pharmaceutical manufacturing sector. This includes revenue generation, product category analysis, and regional penetration, ensuring accuracy through validation against industry standards.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are formulated and then validated via expert consultations, providing insights from senior professionals within the industry. These expert insights refine the analysis and confirm the projected market trajectories.
Step 4: Research Synthesis and Final Output
The synthesis phase compiles data from various sources, including pharmaceutical manufacturing firms and industry experts. This step integrates insights to ensure a comprehensive and verified market analysis output, offering an authoritative perspective on the markets current and future potential.
Frequently Asked Questions
1. How big is the Global Pharmaceutical Manufacturing Market?
The global pharmaceutical manufacturing market is valued at USD 525 billion, supported by ongoing investments in R&D, robust demand for generics, and the growth of biologics manufacturing.
2. What are the challenges in the Global Pharmaceutical Manufacturing Market?
Challenges in the global pharmaceutical manufacturing market include high regulatory compliance costs, supply chain complexity, and the need for continuous technological upgrades in manufacturing processes.
3. Who are the major players in the Global Pharmaceutical Manufacturing Market?
Key players include Pfizer, Novartis, Johnson & Johnson, Roche, and Merck & Co., Inc., each excelling through expansive global reach, innovative R&D, and diversified product lines.
4. What factors are driving growth in the Global Pharmaceutical Manufacturing Market?
Growth in the global pharmaceutical manufacturing market is driven by increasing demand for specialty drugs, advancements in continuous manufacturing technology, and supportive government regulations for drug innovation.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















