
Global Surgical Tourniquet Market Outlook to 2030
Region:Global
Author(s):Vijay Kumar
Product Code:KROD1584
December 2024
92
About the Report
Global Surgical Tourniquet Market Overview
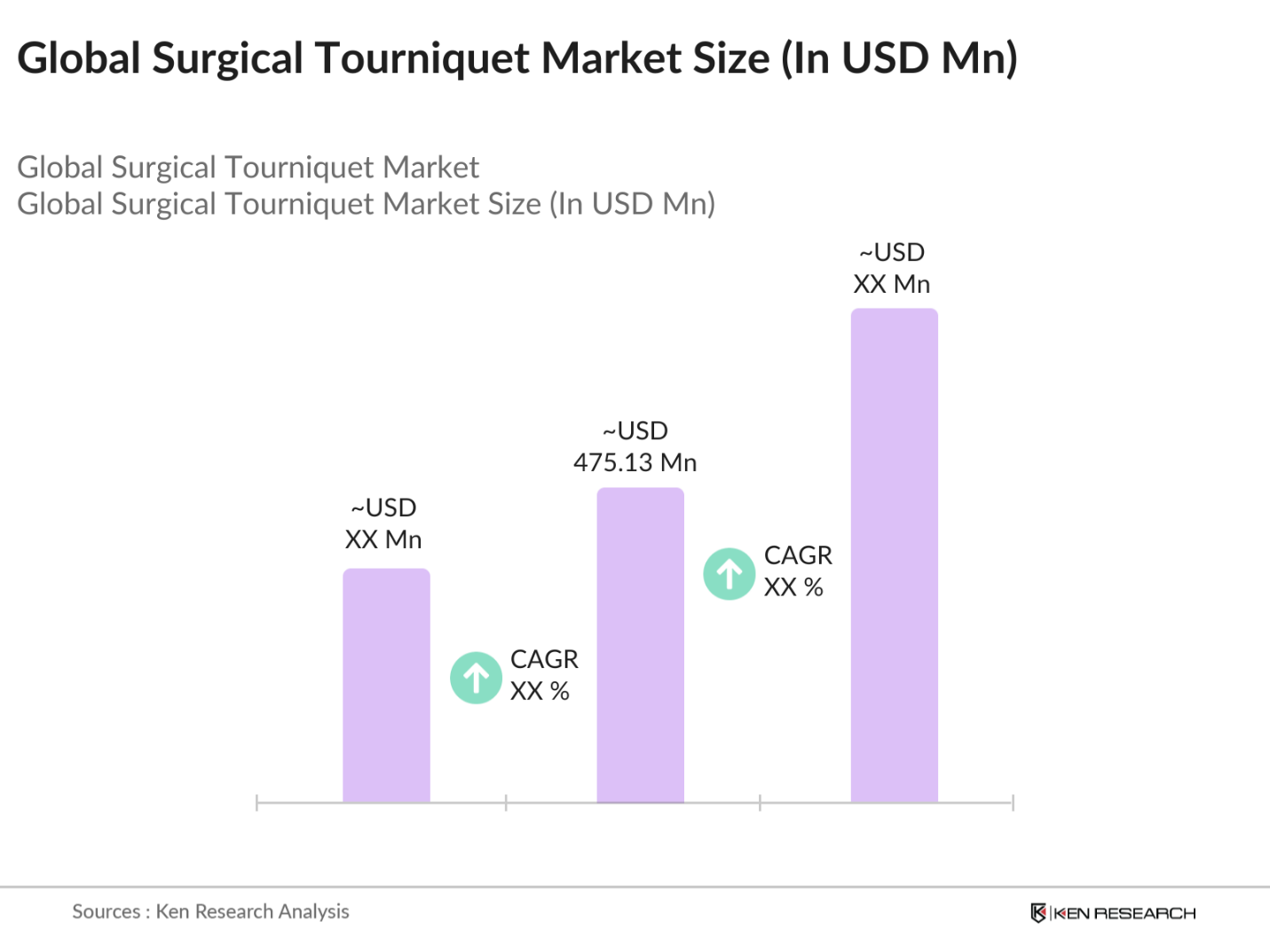
- The global surgical tourniquet market reached a valuation of USD 475.13 million in 2023, driven by the increasing prevalence of orthopedic surgeries, trauma cases, and the adoption of advanced surgical technologies. The market's growth is fueled by rising healthcare expenditures, an aging population leading to more surgical procedures, and innovations in tourniquet systems, including the development of smart and disposable tourniquets. This market is essential in providing critical tools for surgeries where bloodless operating fields are necessary.
- Major players in the global surgical tourniquet market include Zimmer Biomet, Stryker Corporation, Ulrich Medical, Delfi Medical, and Hammarplast Medical. These companies dominate the market through extensive product portfolios, strong R&D capabilities, and strategic acquisitions. Their global distribution networks and focus on continuous product innovation have solidified their leadership positions in the surgical tourniquet industry.
- In 2024, Stryker Corporation expanded its surgical tourniquet product line by introducing a new electronic tourniquet system designed for complex orthopedic surgeries. This system features real-time pressure monitoring and automatic adjustments, enhancing patient safety during surgeries. Additionally, Delfi Medical launched a lightweight, portable tourniquet system aimed at outpatient and ambulatory surgical centers, reflecting the growing trend towards minimally invasive surgeries.
- North America dominates the global surgical tourniquet market, driven by high demand for surgical procedures and advanced healthcare infrastructure. The region's regulatory environment, particularly the U.S. FDAs updated guidelines on surgical tourniquet safety, has also propelled market growth. Europe follows closely, with strong demand from countries like Germany and the UK, where healthcare systems are increasingly adopting disposable tourniquet systems.
Global Surgical Tourniquet Market Segmentation
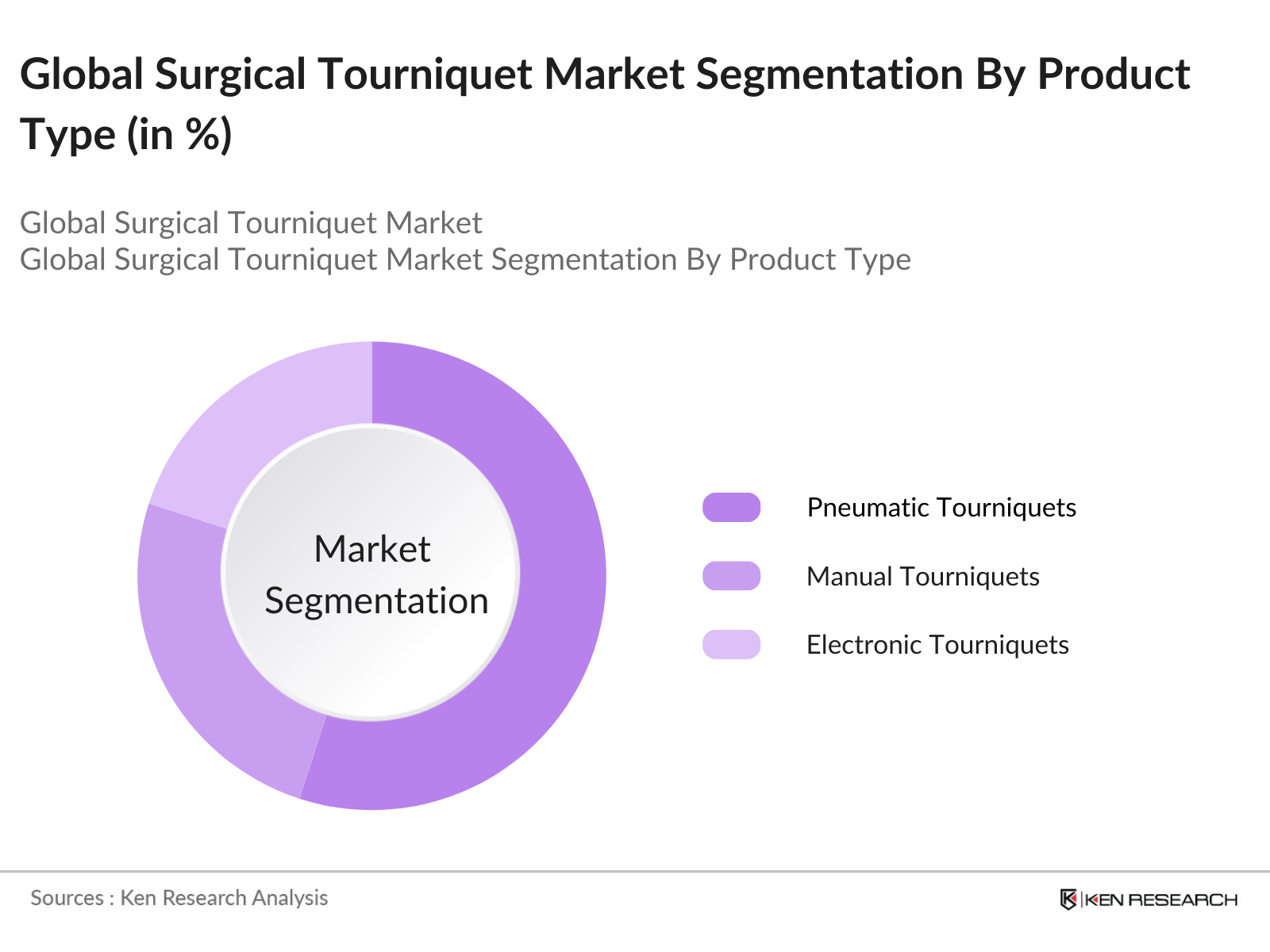
The Global Surgical Tourniquet Market can be segmented based on Product Type, Application, and Region.
By Product Type: The market is segmented by product type into pneumatic tourniquets, manual tourniquets, and electronic tourniquets. In 2023, pneumatic tourniquets held the dominant market share due to their widespread use in orthopedic surgeries. The preference for pneumatic tourniquets stems from their ability to maintain consistent pressure, reducing the risk of complications.
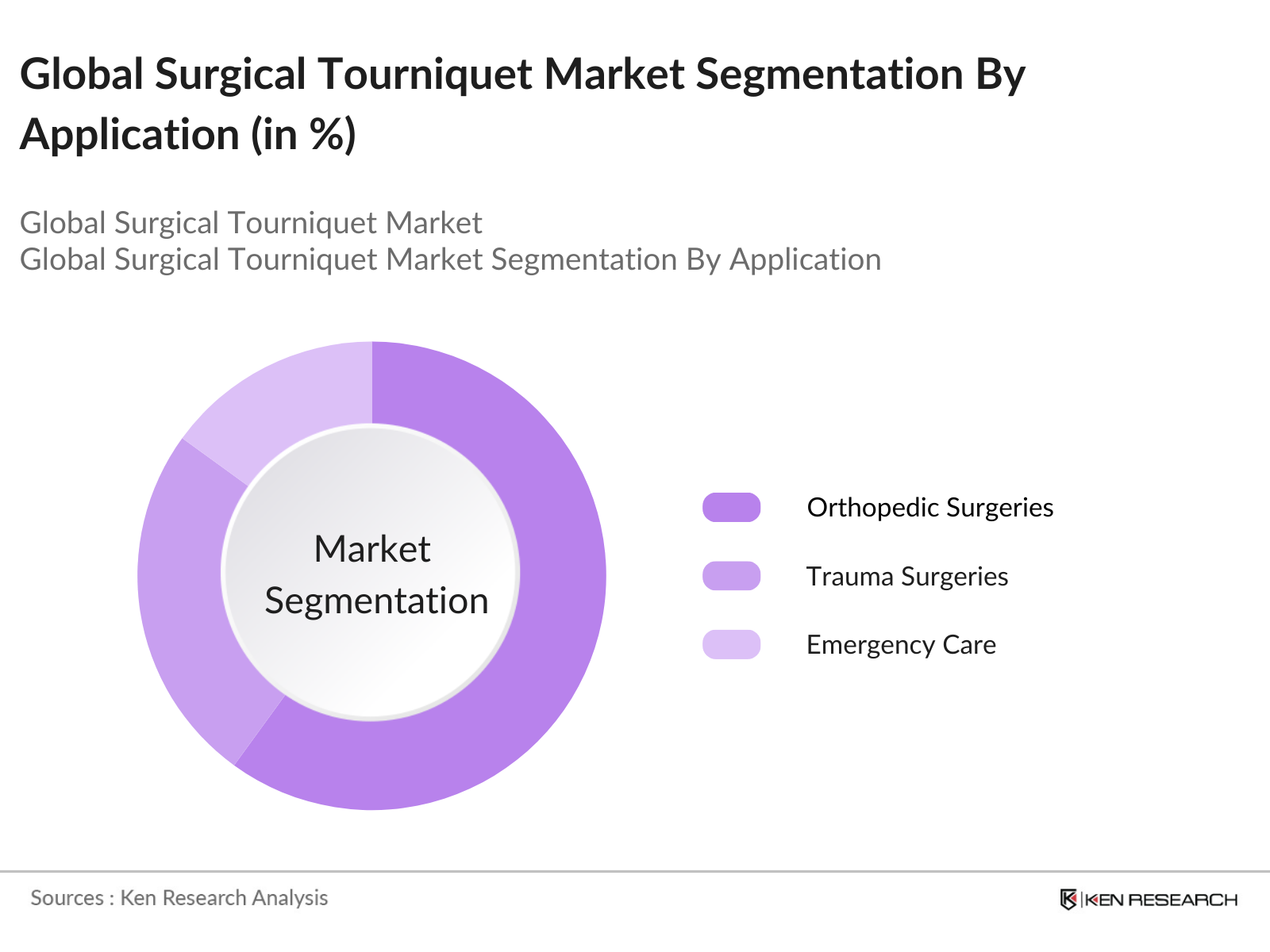
By Application: The market is further segmented by application into orthopedic surgeries, trauma surgeries, and emergency care. In 2023, the orthopedic surgeries segment accounted for the largest market share due to the high number of knee and hip replacement procedures globally. The demand for tourniquets in orthopedic surgeries is driven by the need to create a bloodless surgical field, which is essential for the precision and success of these procedures.
By Region: Geographically, the market is segmented into North America, Europe, Asia-Pacific, Latin America, and MEA. In 2023, North America dominated the market, driven by a high concentration of key players, advanced healthcare infrastructure, and strong demand for surgical procedures. The regions regulatory environment and significant R&D investments have also contributed to its leadership position.
Global Surgical Tourniquet Market Competitive Landscape
|
Company |
Headquarters |
Establishment Year |
|
Zimmer Biomet |
Warsaw, Indiana, USA |
1927 |
|
Stryker Corporation |
Kalamazoo, Michigan, USA |
1941 |
|
Ulrich Medical |
Ulm, Germany |
1912 |
|
Delfi Medical |
Vancouver, Canada |
1988 |
|
Hammarplast Medical |
Lidkping, Sweden |
1967 |
- Zimmer Biomet (2024): Zimmer Biomet launched its latest disposable tourniquet system in 2024, designed for single-use applications to reduce cross-contamination risks in surgical settings. This new product has gained traction in hospitals across Europe and North America, where infection control is a top priority. The companys focus on innovation and safety enhancements has further strengthened its position in the global market.
- Hammarplast Medical: Hammarplast Medical launched theTourniquet 800in2021, a double-channel pneumatic tourniquet featuring a touch screen interface that eliminates physical buttons, thereby enhancing safety. This device is equipped with a NiMH battery backup, ensuring reliable operation even during power outages, which is critical in surgical environments.
Global Surgical Tourniquet Market Analysis
Market Growth Drivers:
- Rising Number of Orthopedic Surgeries: The increasing number of orthopedic surgeries globally is a significant growth driver for the surgical tourniquet market. The demand for tourniquets is particularly high in knee and hip replacement surgeries, where maintaining a bloodless field is crucial. In 2023, over 2.2 million knee replacement surgeries were performed worldwide, with the United States and Europe leading in procedure volumes. This trend is expected to continue, driven by the aging population and the prevalence of musculoskeletal disorders.
- Adoption of Advanced Tourniquet Systems: The adoption of advanced tourniquet systems, including smart and electronic tourniquets, is driving market growth. These systems offer features such as real-time pressure monitoring and automatic adjustments, enhancing patient safety during surgeries. In 2024, the adoption of smart tourniquet systems increased by 15% in North America, reflecting the growing demand for advanced surgical tools that improve outcomes and reduce complications.
- Increasing Awareness and Training: There is a growing emphasis on proper training and awareness in surgical tourniquets, particularly in developing regions. In 2024, WHO launched a global training program aimed at educating healthcare professionals on the safe and effective use of tourniquets. This initiative is expected to reduce the incidence of tourniquet-related complications and improve surgical outcomes in regions where training has been limited.
Global Surgical Tourniquet Market Challenges:
- High Cost of Advanced Systems: The high cost of advanced tourniquet systems remains a significant challenge for the market. In 2024, the average cost of an electronic tourniquet system was recorded at USD 12,000, making it less accessible for smaller hospitals, particularly in developing regions. This cost barrier limits the adoption of these systems, affecting market growth in areas with limited healthcare budgets.
- Regulatory Compliance: Navigating the complex regulatory landscape for medical devices is another challenge for the surgical tourniquet market. In 2023, the European Union implemented new Medical Device Regulation (MDR) standards, increasing the cost and time required to bring new tourniquet products to market. Smaller manufacturers, in particular, face difficulties in meeting these stringent regulatory requirements, which can hinder innovation and market entry.
Global Surgical Tourniquet Market Government Initiatives:
- Indias National Medical Devices Policy (2024): The Government of India launched the National Medical Devices Policy in 2024, aimed at promoting the domestic manufacturing of medical devices, including surgical tourniquets. The policy provides financial incentives and tax breaks to local manufacturers, encouraging them to invest in the production of cost-effective tourniquets. This initiative is expected to boost the growth of the surgical tourniquet market in India by reducing dependency on imports.
- European Unions MDR Implementation (2023): The implementation of the Medical Device Regulation (MDR) in 2023 by the European Union has had a significant impact on the surgical tourniquet market. The MDR requires stricter testing, certification, and post-market surveillance of medical devices, including tourniquets. This regulation has pushed manufacturers to innovate and improve the safety and efficacy of their products, fostering a more competitive and high-quality market environment.
Global Surgical Tourniquet Market Future Outlook
Future Market Trends:
- Expansion of Smart Tourniquet Systems: By 2028, the global surgical tourniquet market is expected to see significant growth, driven by the widespread adoption of smart tourniquet systems. These systems, equipped with advanced sensors and real-time data analytics, will become standard in operating rooms worldwide. The integration of artificial intelligence (AI) and machine learning (ML) into these systems will enable more precise pressure control and personalized settings for each patient, reducing the risk of complications and improving surgical outcomes.
- Increased Use of Disposable Tourniquets: The trend towards single-use medical devices will continue to grow, with disposable tourniquets becoming the preferred choice in most healthcare settings by 2028. This shift will be driven by stringent infection control protocols and the need to reduce hospital-acquired infections (HAIs). As a result, the disposable tourniquet segment is projected to see robust growth, particularly in developed markets such as North America and Europe, where healthcare regulations are increasingly favoring single-use products.
Scope of the Report
|
By Product |
Pneumatic Tourniquets Manual Tourniquets Electronic Tourniquets |
|
By End-User |
Hospitals Ambulatory Surgical Centers (ASCs) Military and Emergency Services |
|
By Region |
North America Europe APAC Latin America MEA |
|
By Application |
Orthopedic Surgeries Trauma Surgeries Emergency Care |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Orthopedic Surgeons
Trauma Care Centers
Hospitals and Surgical Centers
Healthcare Equipment Distributors
Government and Regulatory Bodies (e.g., FDA, EMA)
Medical Device Manufacturers
Investors and Venture Capitalist Firms
Healthcare Industry Associations
Time Period Captured in the Report:
Historical Period: 2018-2023
Base Year: 2023
Forecast Period: 2023-2028
Companies
Players Mentioned in the Report
Zimmer Biomet
Stryker Corporation
Ulrich Medical
Delfi Medical
Hammarplast Medical
Anetic Aid
VBM Medizintechnik GmbH
Pyng Medical Corporation
D & D Technologies
Table of Contents
1. Global Surgical Tourniquet Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. Global Surgical Tourniquet Market Size (in USD Million), 2018-2023
2.1. Historical Market Size
2.2. Year-on-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. Global Surgical Tourniquet Market Analysis
3.1. Growth Drivers
3.1.1. Rising Number of Orthopedic Surgeries
3.1.2. Adoption of Advanced Tourniquet Systems
3.1.3. Increasing Awareness and Training
3.2. Challenges
3.2.1. High Cost of Advanced Systems
3.2.2. Regulatory Compliance
3.2.3. Supply Chain Disruptions
3.3. Opportunities
3.3.1. Technological Advancements and AI Integration
3.3.2. Expansion in Emerging Markets
3.3.3. Increased Demand for Disposable Tourniquets
3.4. Trends
3.4.1. Surge in Adoption of Smart Tourniquet Systems
3.4.2. Shift Towards Single-Use Tourniquets
3.4.3. Growth in Ambulatory Surgical Centers
3.5. Government Regulations
3.5.1. U.S. FDAs Updated Guidelines (2023)
3.5.2. Indias National Medical Devices Policy (2024)
3.5.3. European Unions MDR Implementation (2023)
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem
3.8. Competition Ecosystem
4. Global Surgical Tourniquet Market Segmentation, 2023
4.1. By Product Type (in Value %)
4.1.1. Pneumatic Tourniquets
4.1.2. Manual Tourniquets
4.1.3. Electronic Tourniquets
4.2. By Application (in Value %)
4.2.1. Orthopedic Surgeries
4.2.2. Trauma Surgeries
4.2.3. Emergency Care
4.3. By End-User (in Value %)
4.3.1. Hospitals
4.3.2. Ambulatory Surgical Centers (ASCs)
4.3.3. Military and Emergency Services
4.4. By Region (in Value %)
4.4.1. North America
4.4.2. Europe
4.4.3. Asia-Pacific
4.4.4. Latin America
4.4.5. MEA
5. Global Surgical Tourniquet Market Cross Comparison
5.1. Detailed Profiles of Major Companies
5.1.1. Zimmer Biomet
5.1.2. Stryker Corporation
5.1.3. Ulrich Medical
5.1.4. Delfi Medical
5.1.5. Hammarplast Medical
5.2. Cross Comparison Parameters (No. of Employees, Headquarters, Inception Year, Revenue)
6. Global Surgical Tourniquet Market Competitive Landscape
6.1. Market Share Analysis
6.2. Strategic Initiatives
6.3. Mergers and Acquisitions
6.4. Investment Analysis
6.4.1. Venture Capital Funding
6.4.2. Government Grants
6.4.3. Private Equity Investments
7. Global Surgical Tourniquet Market Regulatory Framework
7.1. Medical Device Safety Standards
7.2. Compliance Requirements
7.3. Certification Processes
8. Global Surgical Tourniquet Market Future Size (in USD Million), 2023-2028
8.1. Future Market Size Projections
8.2. Key Factors Driving Future Market Growth
9. Global Surgical Tourniquet Market Future Segmentation, 2028
9.1. By Product Type (in Value %)
9.2. By Application (in Value %)
9.3. By End-User (in Value %)
9.4. By Region (in Value %)
10. Global Surgical Tourniquet Market Analysts Recommendations
10.1. TAM/SAM/SOM Analysis
10.2. Customer Cohort Analysis
10.3. Marketing Initiatives
10.4. White Space Opportunity Analysis
Disclaimer
Contact Us
Research Methodology
Step 1: Identifying Key Variables:
Ecosystem creation for all the major entities within the global surgical tourniquet market by referring to multiple secondary and proprietary databases. This step involves performing desk research to collate industry-level information and identify key trends and factors influencing the market.
Step 2: Market Building:
Collating statistics on the global surgical tourniquet market over the years, including the penetration of product types and service providers, to compute the revenue generated within the market. This process also involves reviewing service quality statistics to ensure the accuracy and reliability of the data points shared, providing a robust understanding of market revenue dynamics.
Step 3: Validating and Finalizing:
Building market hypotheses and conducting Computer Assisted Telephone Interviews (CATIs) with industry experts from various companies within the surgical tourniquet market. These interviews are designed to validate the collected statistics, refine market forecasts, and obtain operational and financial insights directly from company representatives.
Step 4: Research Output:
Our team will engage with multiple key surgical tourniquet companies to understand the nature of product segments, sales patterns, consumer preferences, and other relevant parameters. This approach will support the validation of statistics derived through a bottom-up approach, ensuring that the final research output accurately reflects the market conditions and supports strategic decision-making.
Frequently Asked Questions
1. How big is the Global Surgical Tourniquet Market?
The global surgical tourniquet market reached a valuation of USD 475.13 million in 2023, driven by the increasing prevalence of orthopedic surgeries, trauma cases, and the adoption of advanced surgical technologies.
2. What are the challenges in the Global Surgical Tourniquet Market?
Challenges in the global surgical tourniquet market include the high cost of advanced tourniquet systems, stringent regulatory compliance requirements, and supply chain disruptions affecting the availability of key components necessary for manufacturing.
3. Who are the major players in the Global Surgical Tourniquet Market?
Key players in the global surgical tourniquet market include Zimmer Biomet, Stryker Corporation, Ulrich Medical, Delfi Medical, and Hammarplast Medical. These companies lead the market due to their innovative product offerings, extensive distribution networks, and strong focus on research and development.
4. What are the growth drivers of the Global Surgical Tourniquet Market?
The market is driven by the rising number of orthopedic surgeries, increasing adoption of advanced tourniquet systems, and growing awareness and training on the proper use of surgical tourniquets, particularly in developing regions.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















