
India Endoscopic Devices Market Outlook to 2030
Region:Asia
Author(s):Yogita Sahu
Product Code:KROD6899
November 2024
89
About the Report
India Endoscopic Devices Market Overview
- The India Endoscopic Devices Market is valued at USD 1.2 billion. This market is driven by several factors, including an increasing demand for minimally invasive surgeries, advancements in endoscopic technologies, and a growing geriatric population that is more susceptible to conditions requiring endoscopic procedures.
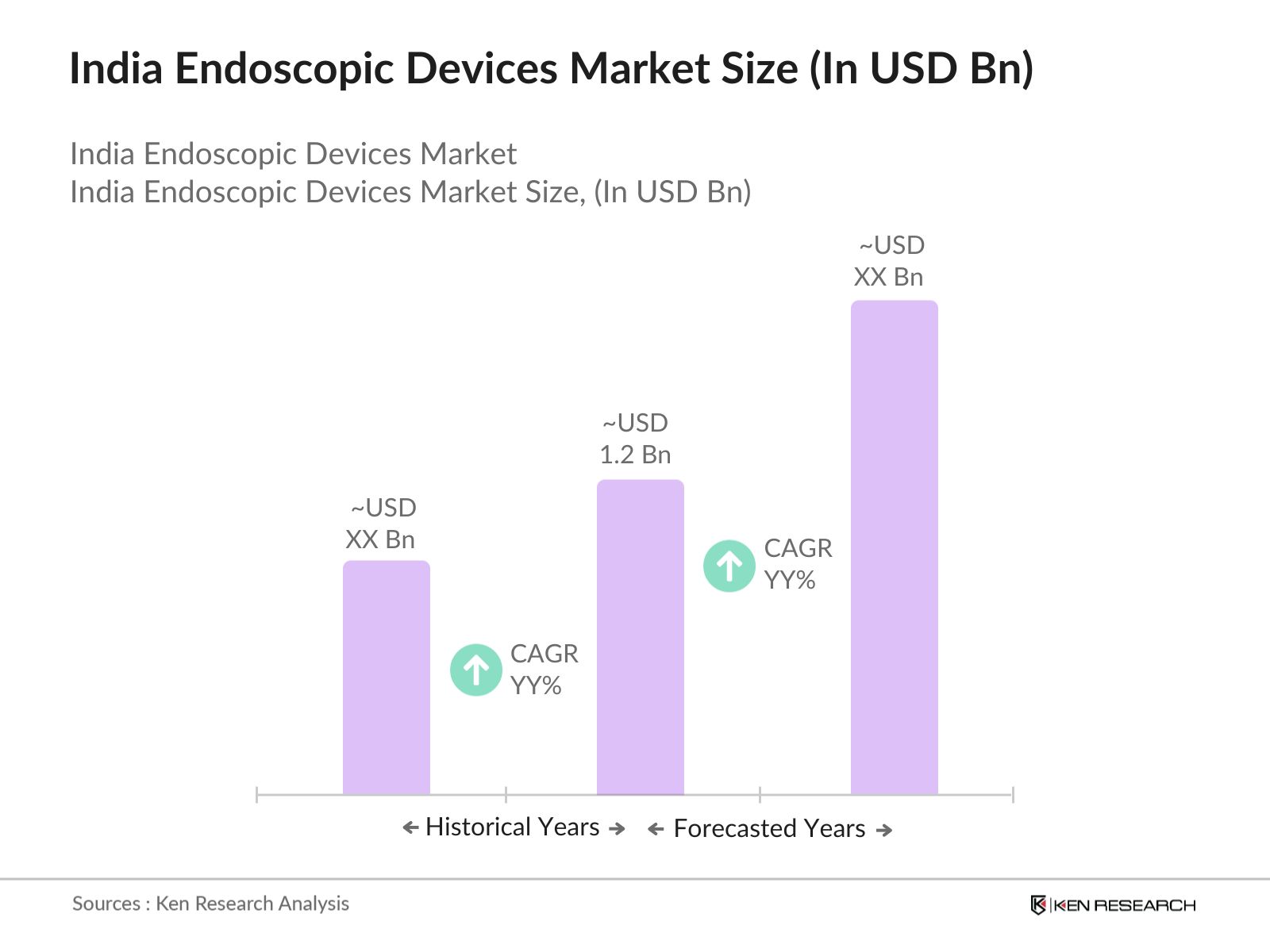
- In terms of geographical dominance, major cities like Mumbai, Delhi, and Bangalore drive the market due to the concentration of well-established hospitals, healthcare centers, and medical institutions. These cities benefit from superior healthcare infrastructure, access to advanced medical technologies, and a higher demand for elective surgeries that often require endoscopic procedures.
- In 2023, the Indian government introduced a PLI scheme, allocating INR 15,000 crore to medical device manufacturers, including those specializing in endoscopic technologies. This has led to a surge in local production, with 30+ companies receiving financial support to produce high-quality endoscopic devices domestically, reducing dependence on imports.
India Endoscopic Devices Market Segmentation
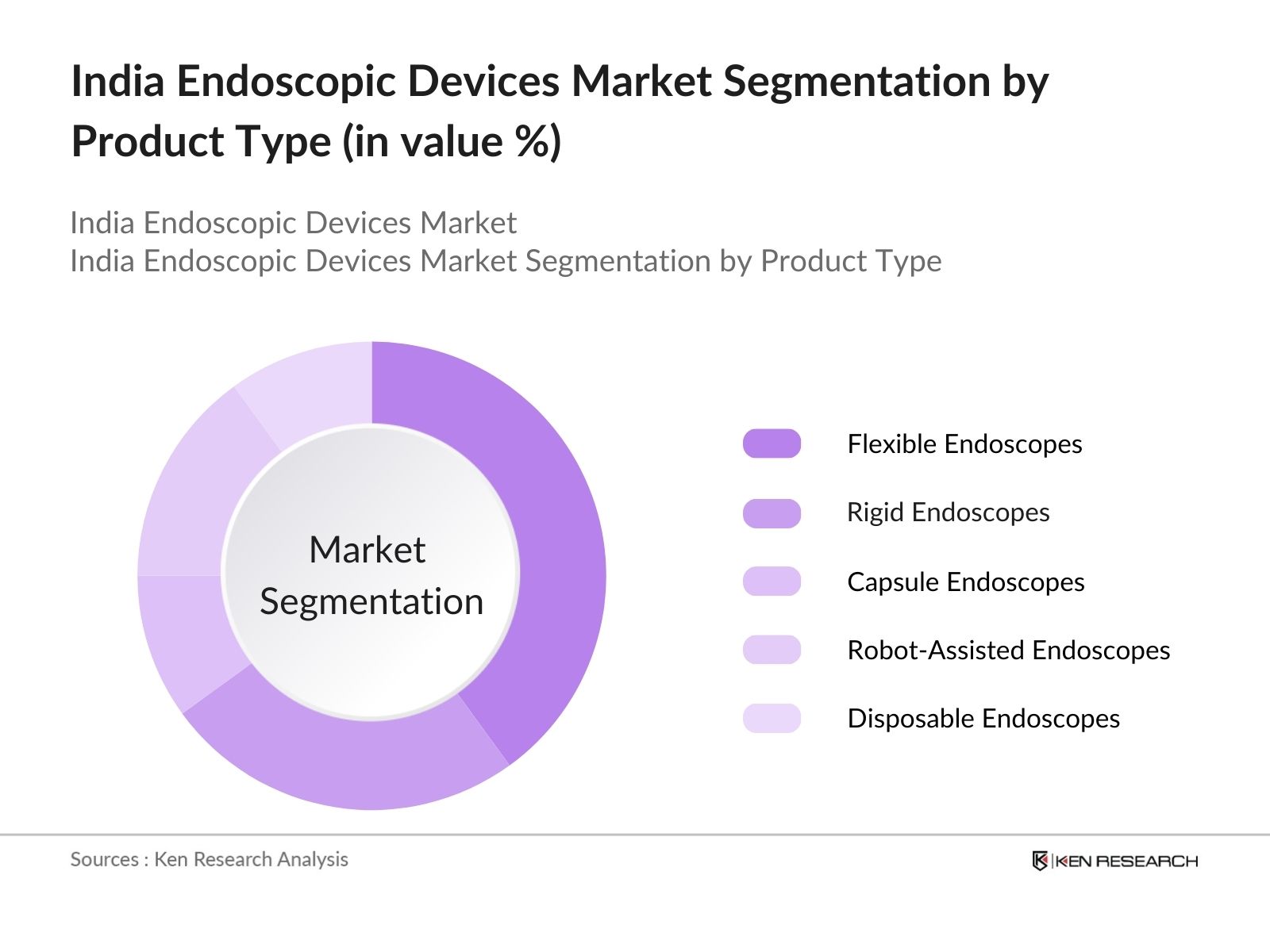
By Product Type: The market is segmented by product type into flexible endoscopes, rigid endoscopes, capsule endoscopes, robot-assisted endoscopes, and disposable endoscopes. Flexible endoscopes hold a dominant market share due to their versatility in performing multiple procedures, particularly in gastroenterology. These devices are favored because they allow physicians to access difficult-to-reach areas within the body while offering superior imaging quality.
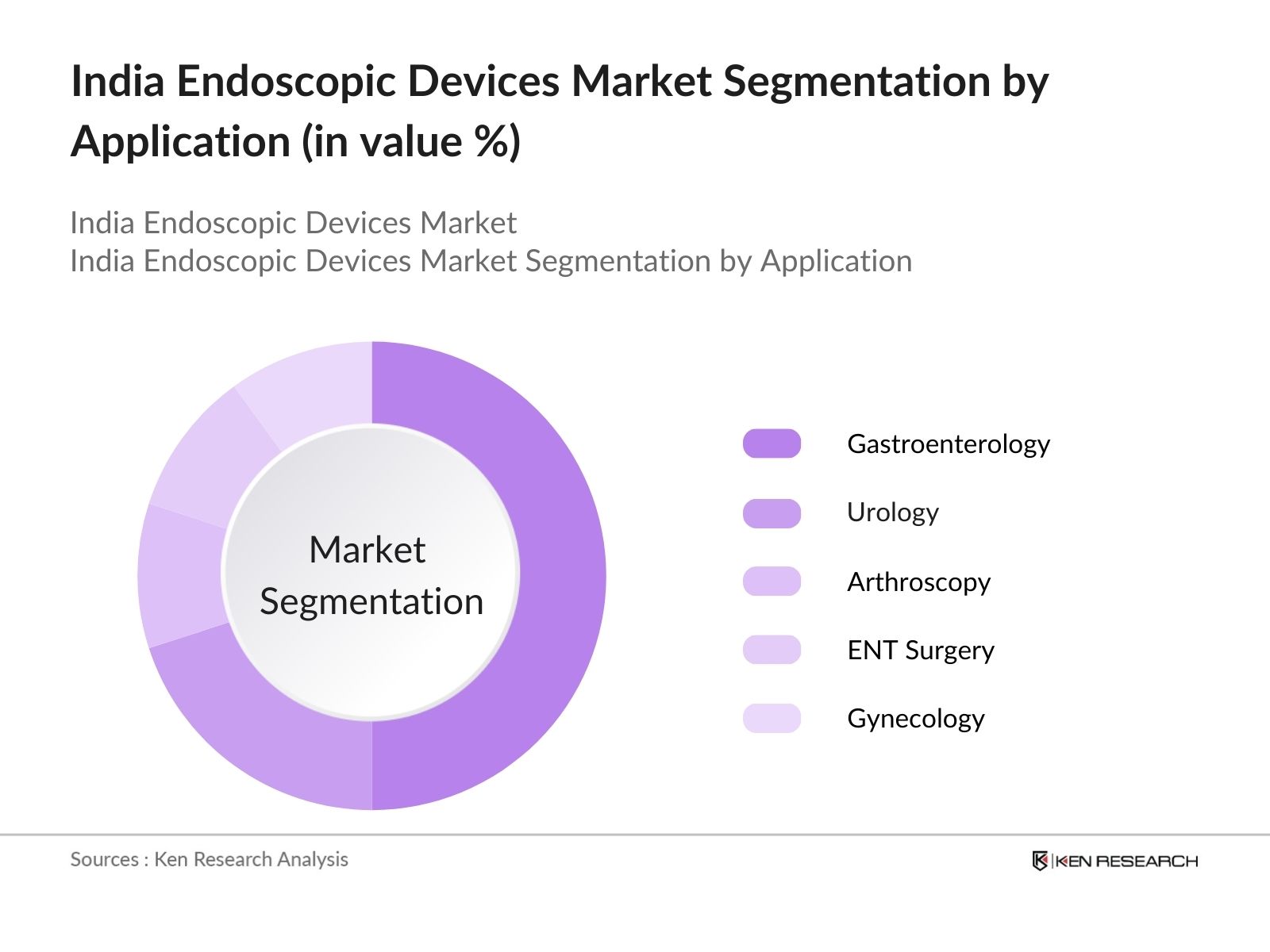
By Application: The market is also segmented by application into gastroenterology, urology, arthroscopy, ENT surgery, and gynecology. Gastroenterology has emerged as the dominant application segment due to the rising incidence of gastrointestinal disorders, such as colorectal cancer and Crohns disease, which require endoscopic interventions. The increasing preference for minimally invasive surgeries in the gastrointestinal space, combined with the rising number of early-stage screenings, contributes significantly to this segment's market dominance.
India Endoscopic Devices Market Competitive Landscape
The market is characterized by the presence of several international and domestic players. Companies like Olympus, Karl Storz, and Fujifilm dominate the market, with significant investments in research and development and advanced manufacturing facilities.
|
Company |
Establishment Year |
Headquarters |
Global Presence |
Product Portfolio |
R&D Spending |
Revenue |
Market Strategy |
Acquisition History |
Key Patents |
|
Olympus Corporation |
1919 |
Tokyo, Japan |
|||||||
|
Karl Storz SE & Co. KG |
1945 |
Tuttlingen, Germany |
|||||||
|
Fujifilm Holdings Corporation |
1934 |
Tokyo, Japan |
|||||||
|
Stryker Corporation |
1941 |
Michigan, USA |
|||||||
|
Boston Scientific |
1979 |
Marlborough, USA |
India Endoscopic Devices Market Analysis
Market Growth Drivers
- Increasing Prevalence of Chronic Diseases: In 2024, India reports over 77 million diabetes patients and nearly 30 million people with cancer, driving the need for minimally invasive surgeries. Endoscopic devices play a crucial role in early diagnosis and treatment of these diseases. Hospitals are increasingly investing in endoscopic technologies to meet this growing demand, with 5,000+ hospitals across India purchasing these devices for their diagnostic capabilities.
- Rising Geriatric Population: Indias population aged 60 and above is projected to reach 140 million by 2024, leading to an increase in age-related diseases such as gastrointestinal, orthopedic, and respiratory conditions. These conditions require frequent endoscopic interventions, fueling the demand for advanced devices in both private and public hospitals.
- Expansion of Healthcare Infrastructure: The Indian government allocated INR 86,000 crore for healthcare infrastructure in the 2023 budget, improving the capacity of tertiary care centers. This includes enhancing hospitals ability to perform endoscopic surgeries, with 800+ new hospitals established under public-private partnerships in tier 2 and tier 3 cities, expanding the reach of endoscopic devices in rural areas.
Market Challenges
- Limited Skilled Professionals: India currently faces a shortfall of 1.8 million healthcare professionals, which restricts the ability to operate advanced endoscopic devices in smaller healthcare facilities. Lack of training programs for endoscopic surgery in over 40% of medical institutions in rural regions further compounds the issue, hindering market growth.
- Stringent Regulatory Approvals: The Central Drugs Standard Control Organization (CDSCO) has implemented stricter protocols for the approval of endoscopic devices. In 2023, around 150 new device models were delayed due to regulatory bottlenecks, leading to extended timelines for product launches and reducing the influx of innovative technologies into the Indian market.
India Endoscopic Devices Market Future Outlook
Over the next five years, the India Endoscopic Devices industry is expected to exhibit robust growth, driven by rising healthcare spending, advancements in medical technology, and an increasing preference for non-invasive surgical procedures.
Market Future Opportunities
- Growth in AI-Based Diagnostic Endoscopy: In the next 5 years, the Indian market will see a surge in AI-based endoscopic systems for early disease detection. Over 10,000 AI-powered endoscopy units are anticipated to be installed by 2028, improving diagnostic accuracy and enabling faster interventions for critical diseases.
- Increased Adoption of Capsule Endoscopy: Capsule endoscopy will see significant growth, with over 100,000 procedures expected to be performed by 2027. This non-invasive diagnostic technique is particularly suited for patients who are unable to undergo traditional endoscopy, making it a preferred choice in specialized medical centers.
Scope of the Report
|
Product Type |
Flexible Endoscopes Rigid Endoscopes Capsule Endoscopes Robot-Assisted Endoscopes Disposable Endoscopes |
|
Application |
Gastroenterology Urology Arthroscopy ENT Surgery Gynecology |
|
End User |
Hospitals Ambulatory Surgical Centers (ASCs) Specialty Clinics Diagnostic Centers |
|
Technology |
Optical Endoscopes Digital Endoscopes Virtual Endoscopy |
|
Region |
North India South India East India West India |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Hospitals and Healthcare Providers
Medical Device Manufacturers
Private Equity Firms
Government and Regulatory Bodies (CDSCO, Ministry of Health & Family Welfare)
Investors and Venture Capitalist Firms
Banks and Financial Institution
Companies
Players Mentioned in the Report:
Olympus Corporation
Karl Storz SE & Co. KG
Fujifilm Holdings Corporation
Boston Scientific Corporation
Stryker Corporation
Medtronic PLC
Johnson & Johnson (Ethicon)
HOYA Corporation (PENTAX Medical)
Smith & Nephew PLC
Richard Wolf GmbH
Table of Contents
India Endoscopic Devices Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (Demand-Supply Equilibrium, Technological Adoption Rate)
1.4. Market Segmentation Overview
India Endoscopic Devices Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis (Units Sold, Devices Installed)
2.3. Key Market Developments and Milestones (Device Registration, Regulatory Approvals)
India Endoscopic Devices Market Analysis
3.1. Growth Drivers
3.1.1. Rising Incidence of Gastrointestinal Disorders
3.1.2. Government Initiatives for Healthcare Infrastructure
3.1.3. Increasing Minimally Invasive Surgeries
3.1.4. Healthcare Insurance Penetration
3.2. Market Challenges
3.2.1. High Costs of Advanced Devices
3.2.2. Lack of Skilled Workforce in Rural Areas
3.2.3. Regulatory Approval Delays
3.2.4. Low Awareness in Tier-2 and Tier-3 Cities
3.3. Opportunities
3.3.1. Expansion of Healthcare Infrastructure in Tier-2 Cities
3.3.2. Collaboration with Global Technology Firms
3.3.3. Growing Medical Tourism Industry
3.3.4. Increasing R&D for Advanced Endoscopic Solutions
3.4. Trends
3.4.1. Adoption of AI-Assisted Endoscopic Procedures
3.4.2. Disposable Endoscopes for Infection Control
3.4.3. Integration of Robotic Surgery with Endoscopy
3.4.4. Remote Endoscopic Monitoring for Diagnostics
3.5. Government Regulation
3.5.1. Medical Device Regulations (MD Rules) Compliance
3.5.2. FDI Policies for Medical Device Manufacturing
3.5.3. Certification Standards for Medical Devices (CE Marking, FDA Approval)
3.5.4. Subsidies for Hospital Upgrades (Focus on Endoscopic Procedures)
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem (Endoscopy Device Manufacturers, Healthcare Providers, Distributors, Regulators)
3.8. Porters Five Forces Analysis
3.9. Competitive Ecosystem
India Endoscopic Devices Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Flexible Endoscopes
4.1.2. Rigid Endoscopes
4.1.3. Capsule Endoscopes
4.1.4. Robot-Assisted Endoscopes
4.1.5. Disposable Endoscopes
4.2. By Application (In Value %)
4.2.1. Gastroenterology
4.2.2. Urology
4.2.3. Arthroscopy
4.2.4. ENT Surgery
4.2.5. Gynecology
4.3. By End User (In Value %)
4.3.1. Hospitals
4.3.2. Ambulatory Surgical Centers (ASCs)
4.3.3. Specialty Clinics
4.3.4. Diagnostic Centers
4.4. By Technology (In Value %)
4.4.1. Optical Endoscopes
4.4.2. Digital Endoscopes
4.4.3. Virtual Endoscopy
4.5. By Region (In Value %)
4.5.1. North India
4.5.2. South India
4.5.3. East India
4.5.4. West India
India Endoscopic Devices Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Olympus Corporation
5.1.2. Karl Storz SE & Co. KG
5.1.3. Fujifilm Holdings Corporation
5.1.4. Boston Scientific Corporation
5.1.5. Stryker Corporation
5.1.6. Medtronic PLC
5.1.7. Johnson & Johnson (Ethicon)
5.1.8. HOYA Corporation (PENTAX Medical)
5.1.9. Smith & Nephew PLC
5.1.10. Richard Wolf GmbH
5.1.11. Cook Medical
5.1.12. B. Braun Melsungen AG
5.1.13. Conmed Corporation
5.1.14. EndoMed Systems GmbH
5.1.15. Schlly Fiberoptic GmbH
5.2. Cross Comparison Parameters (Revenue, Product Innovation, R&D Spend, Global Presence, Manufacturing Capability, Product Portfolio, Service Contracts, Acquisition History)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers And Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
India Endoscopic Devices Market Regulatory Framework
6.1. Medical Device Standards and Certifications (ISO 13485, MDR)
6.2. Import-Export Policies for Medical Devices
6.3. Licensing and Compliance Requirements
6.4. Quality Control and Safety Standards
India Endoscopic Devices Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth (Healthcare Expansion, Technological Innovations)
India Endoscopic Devices Future Market Segmentation
8.1. By Product Type (In Value %)
8.2. By Application (In Value %)
8.3. By End User (In Value %)
8.4. By Technology (In Value %)
8.5. By Region (In Value %)
India Endoscopic Devices Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
In this step, a comprehensive review of the endoscopic devices ecosystem in India was conducted. This included identifying key stakeholders such as manufacturers, distributors, healthcare providers, and regulators. Secondary research, using reliable industry databases and proprietary sources, helped identify critical variables influencing the market.
Step 2: Market Analysis and Construction
Historical data on endoscopic device sales, usage rates in different healthcare facilities, and demand dynamics were analyzed to understand the growth patterns. The market's revenue generation and penetration levels across various healthcare institutions were also evaluated.
Step 3: Hypothesis Validation and Expert Consultation
Market assumptions were verified through in-depth interviews with industry experts, including endoscopy specialists, manufacturers, and hospital procurement heads. These consultations helped refine market forecasts and provided practical insights into operational challenges and opportunities.
Step 4: Research Synthesis and Final Output
A combination of bottom-up and top-down approaches was employed to validate the market size and segmentation. The final analysis was derived from inputs from leading manufacturers, along with real-time data on product usage and healthcare trends across India.
Frequently Asked Questions
01. How big is the India Endoscopic Devices Market?
The India Endoscopic Devices Market is valued at USD 1.2 billion, driven by the increasing demand for minimally invasive surgeries and advancements in endoscopic technology.
02. What are the challenges in the India Endoscopic Devices Market?
Challenges in the India Endoscopic Devices Market include the high cost of advanced devices, regulatory approval delays, and a lack of skilled professionals in rural areas, limiting market penetration.
03. Who are the major players in the India Endoscopic Devices Market?
Key players in the India Endoscopic Devices Market include Olympus Corporation, Karl Storz SE & Co. KG, Fujifilm Holdings Corporation, Boston Scientific Corporation, and Stryker Corporation, known for their technological advancements and extensive product portfolios.
04. What are the growth drivers of the India Endoscopic Devices Market?
Growth drivers in the India Endoscopic Devices Market include the rising prevalence of gastrointestinal disorders, increasing government healthcare expenditure, and the expansion of healthcare infrastructure in both urban and rural areas.
05. Which product types dominate the India Endoscopic Devices Market?
Flexible endoscopes hold the largest share due to their wide applicability in gastroenterology, urology, and gynecology, making them a preferred choice for hospitals and diagnostic centers.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















