
Indonesia Oncology Clinical Trials Market Outlook to 2030
Region:Asia
Author(s):Samanyu
Product Code:KROD962
July 2024
100
About the Report
Indonesia Oncology Clinical Trials Market Overview
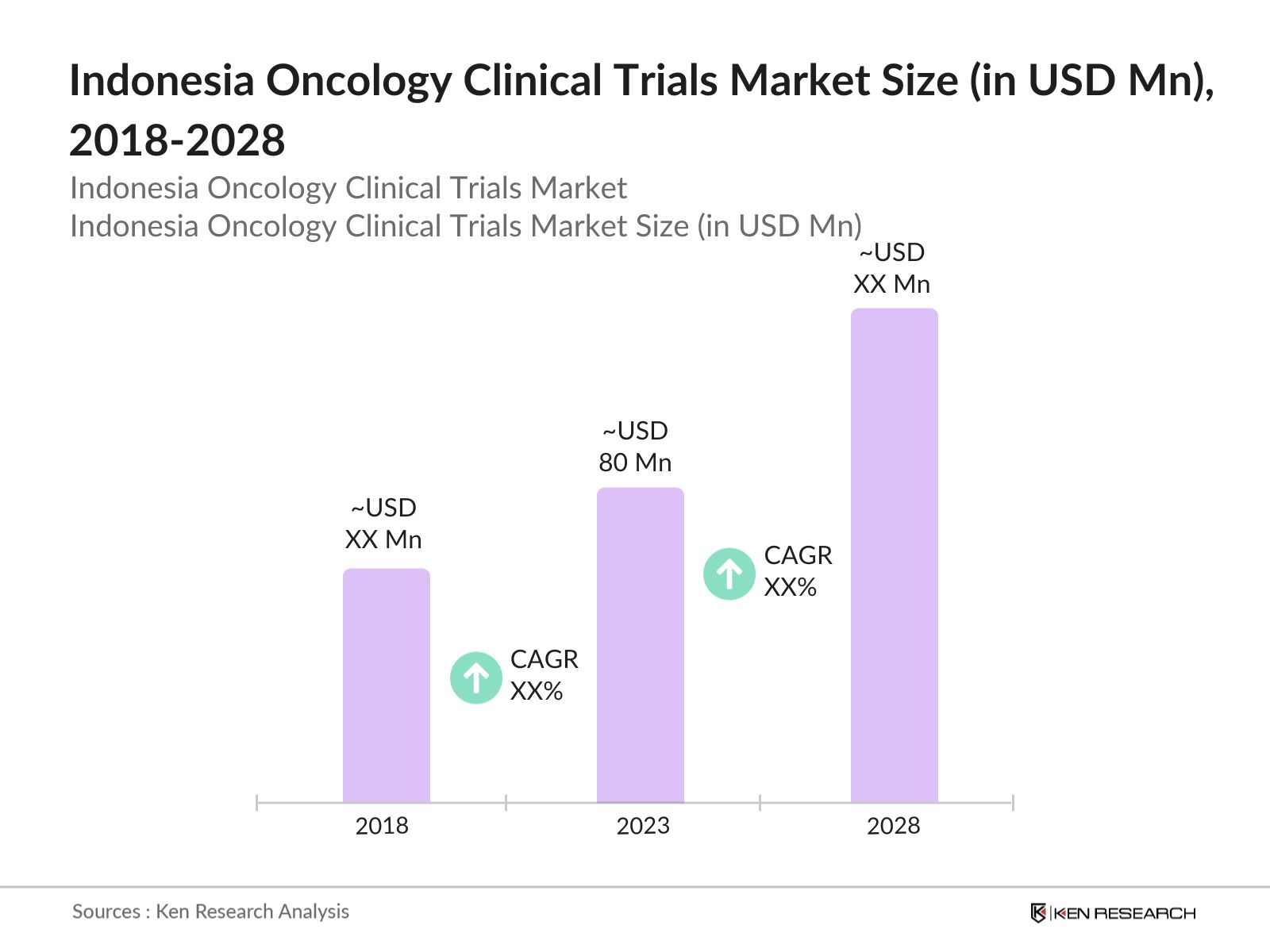
- The Indonesia oncology clinical trials market, valued at USD 80 Mn in 2023, is driven by rising incidence of cancer, increased funding for clinical trials, and the adoption of innovative technologies in cancer treatment. The market's expansion is further fueled by an increasing number of oncology clinical trial centers and improved healthcare infrastructure.
- Key players in the Indonesia Oncology Clinical Trials Market include PT Kalbe Farma Tbk, Prodia Clinical Laboratory, Siloam International Hospitals, Mayapada Hospital Group, and Mochtar Riady Comprehensive Cancer Centre (MRCCC). These companies are pivotal in driving advancements in cancer research and treatment, contributing significantly to the market's growth.
- Not much trials were achieved due to factors such as limited awareness, logistical issues, and cultural barriers. This shortfall highlights the need for targeted outreach programs, improved patient education, and streamlined trial processes to enhance participant engagement and retention.
- Jakarta, the capital city of Indonesia, is the dominant region in the oncology clinical trials market. The city's dominance is attributed to its advanced healthcare infrastructure, concentration of leading medical institutions, and a higher number of specialized oncology centers. Additionally, Jakarta's robust research ecosystem and access to a large patient pool make it an ideal location for conducting clinical trials.
Indonesia Oncology Clinical Trials Market Segmentation
The Indonesia oncology clinical trials market can be segmented based on several factors:
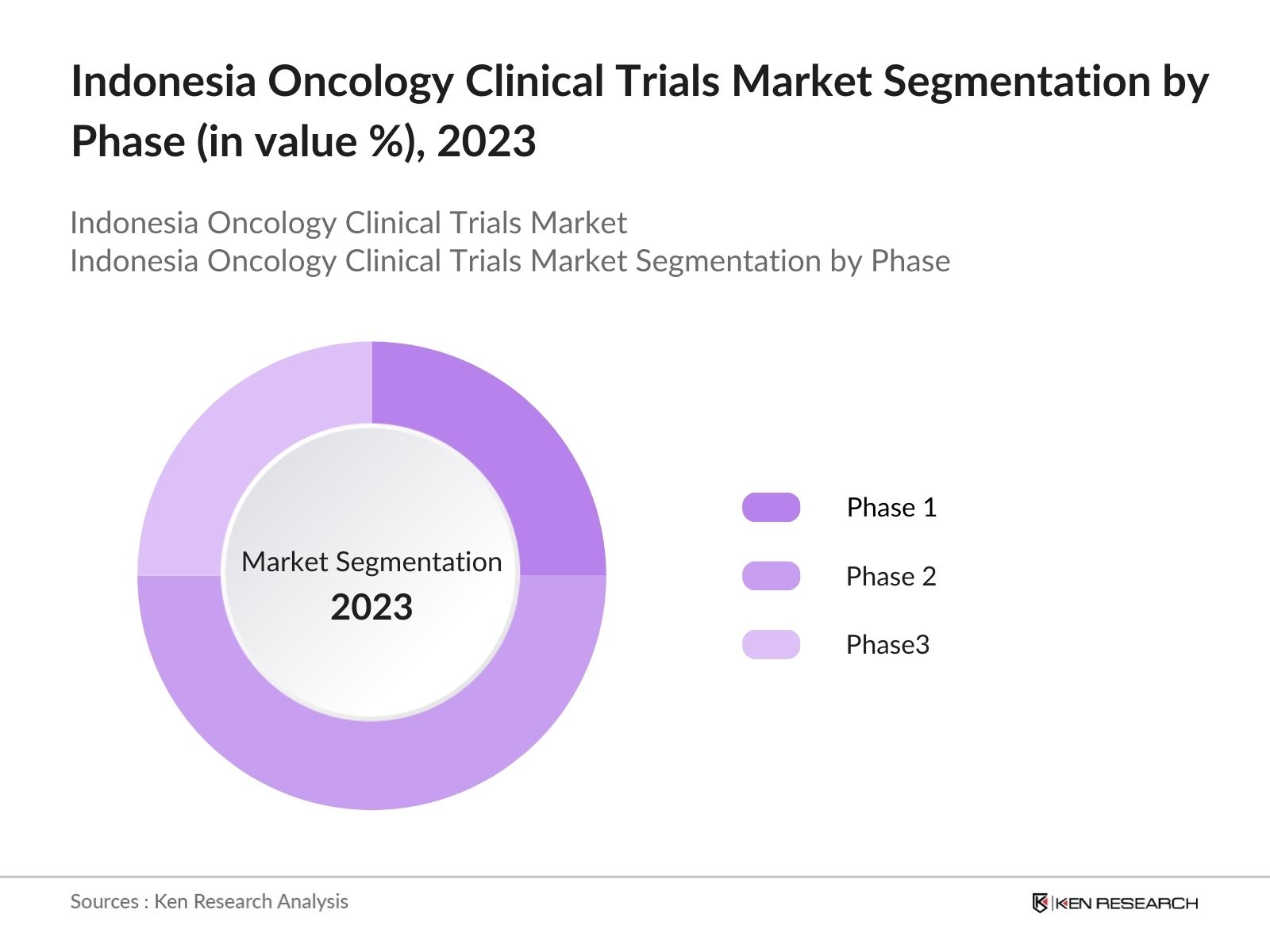
By Phase: Indonesia oncology clinical trials market segmentation by phase is divided into phase 1, phase 2 and phase 3. In 2023, dominance of Phase II trials is driven by the increasing number of experimental drugs advancing through early-stage trials, requiring extensive testing to determine optimal dosages and treatment regimens.
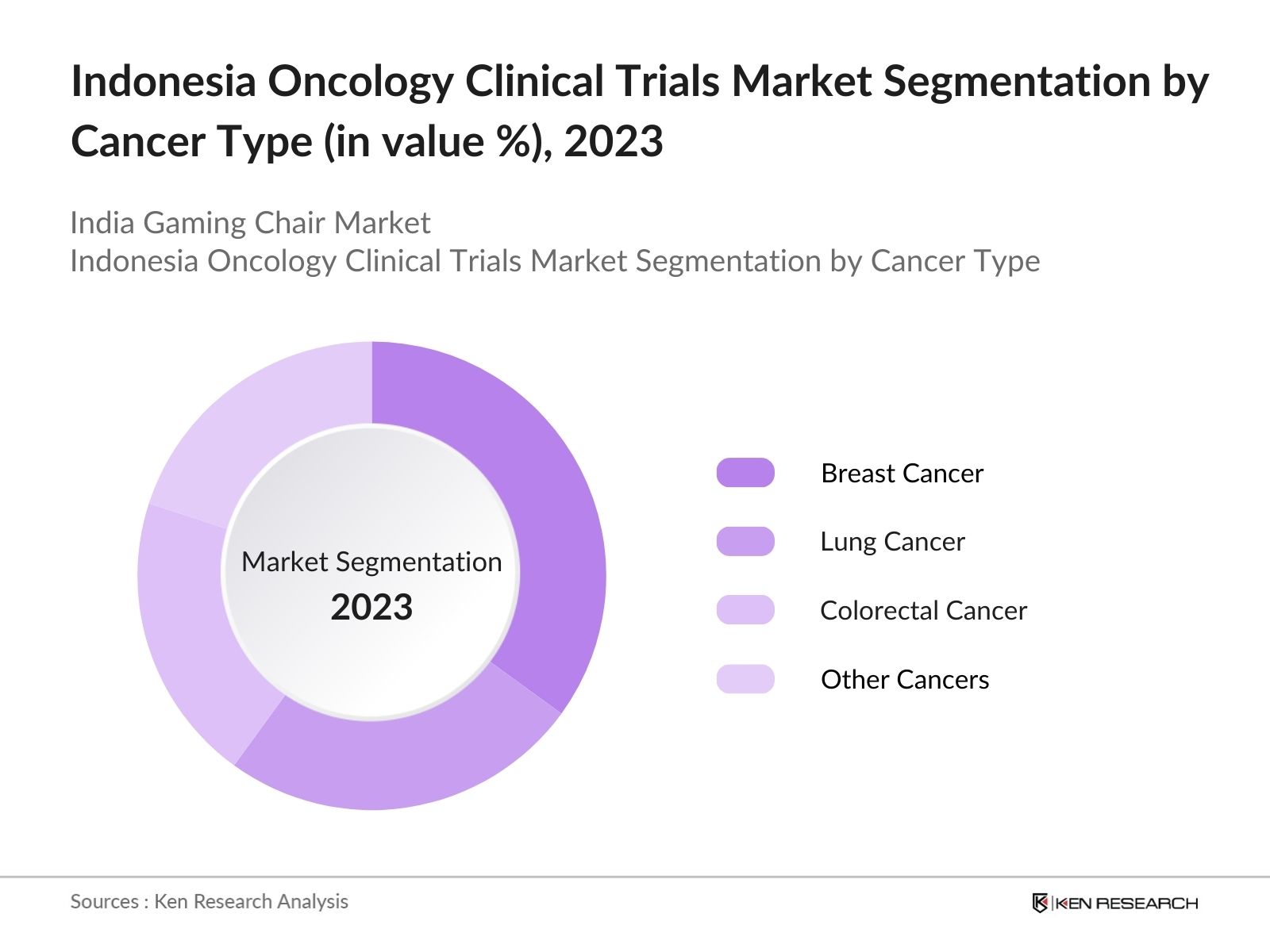
By Cancer Type: Indonesia oncology clinical trials market segmentation by cancer type is divided into Breast Cancer, lung cancer, colorectal cancer and other cancer. In 2023, the dominance of breast cancer trials is attributed to the significant incidence rate of breast cancer in Indonesia. This high prevalence necessitates continuous research and development of effective treatment options.
By Region: Indonesia oncology clinical trials market segmentation by region is divided into north, south, east and west. In 2023, dominance in the north region is attributed to its robust research ecosystem, access to a large patient pool, and the presence of specialized oncology centers. The city's infrastructure supports efficient clinical trial conduct, making it a preferred location for research activities.
Indonesia Oncology Clinical Trials Market Competitive Landscape
|
Company |
Establishment Year |
Headquarters |
|
PT Kalbe Farma Tbk |
1966 |
Jakarta |
|
Prodia Clinical Laboratory |
1973 |
Jakarta |
|
Siloam International Hospitals |
1996 |
Tangerang |
|
Mayapada Hospital Group |
2008 |
Jakarta |
|
Mochtar Riady Comprehensive Cancer Centre (MRCCC) |
2010 |
Jakarta |
- Kalbe Farma and Global Pharma Partnership: In 2023, PT Kalbe Farma Tbk announced a collaboration with a leading global pharmaceutical company to conduct Phase III clinical trials for a novel immunotherapy drug for advanced-stage lung cancer. This partnership represents a significant advancement in bringing cutting-edge cancer treatments to the Indonesian market.
- Siloam International Hospitals' Clinical Trial Management System: In 2024, Siloam International Hospitals introduced an innovative clinical trial management system developed in collaboration with a leading global technology firm. This system is expected to improve the efficiency of clinical trials and support the growing demand for oncology research in Indonesia.
Indonesia Oncology Clinical Trials Industry Analysis
Indonesia Oncology Clinical Trials Market Growth Drivers:
- Increasing Cancer Incidence: In 2024, Indonesia is projected to report over 360,000 new cancer cases, up from 348,000 in 2023. The rising cancer incidence is driving the need for innovative treatment options and clinical trials. As more patients seek advanced therapies, the demand for oncology clinical trials continues to grow, ensuring the development and availability of new treatments for various types of cancer
- Expanding Clinical Trial Networks: Indonesia's clinical trial network has expanded significantly, with over 200 oncology trials conducted in 2023, up from 150 in 2022. This growth is supported by collaborations between local research institutions and international pharmaceutical companies, enhancing the country's capacity to conduct high-quality clinical trials and attract more research investments.
- Rising Healthcare Expenditure: Indonesia's healthcare expenditure is expected to increase. This rise in healthcare spending is attributed to government initiatives and private sector investments aimed at improving healthcare infrastructure and access to advanced medical treatments, including oncology clinical trials.
Indonesia Oncology Clinical Trials Market Challenges:
- Limited Access to Advanced Technology: Many clinical trial centers in Indonesia lack access to advanced diagnostic and treatment technologies, impacting the quality and efficiency of oncology trials. In 2023, few of trial centers were equipped with state-of-the-art technology, highlighting the need for increased investments in medical infrastructure to support high-quality clinical research.
- Regulatory Hurdles: The regulatory approval process for clinical trials in Indonesia can be lengthy and complex, often taking 12-18 months for full approval. This delay hinders the timely initiation of trials and can discourage international sponsors from conducting research in the country. Streamlining regulatory processes is crucial to overcoming this challenge and attracting more clinical trials.
Indonesia Oncology Clinical Trials Market Government Initiatives:
- National Cancer Control Program: In 2023, the Indonesian government launched the National Cancer Control Program, allocating IDR 1 Tn to enhance cancer prevention, early detection, and treatment. This initiative includes substantial funding for oncology clinical trials, supporting the development of new therapies and improving trial infrastructure.
- Tax Incentives for Clinical Research: The government introduced tax incentives in 2024 to encourage private sector investment in clinical research. Companies investing in oncology clinical trials can benefit from reduced corporate tax rates, making it financially attractive to sponsor and conduct research in Indonesia.
Indonesia Oncology Clinical Trials Future Market Outlook
The Indonesia Oncology Clinical Trials Market is expected to show significant growth driven by continuous advancements in cancer research, increasing patient participation in clinical trials, and government support for healthcare innovations. The focus will be on precision medicine, targeted therapies, and personalized treatment approaches, significantly improving patient outcomes and driving market growth.
Future Market Trends
-
- Expansion of Personalized Medicine: Personalized medicine is expected to become a cornerstone of oncology clinical trials in Indonesia. Advances in genomic profiling and targeted therapies will enable the development of customized treatment plans for individual patients, improving efficacy and reducing adverse effects.
- Increased Use of Digital Health Technologies: The adoption of digital health technologies, such as electronic health records (EHRs) and telemedicine, will play a crucial role in the future of oncology clinical trials. These technologies will streamline trial processes, enhance patient monitoring, and improve data collection and analysis.
Scope of the Report
|
By Phase |
Phase 1 Phase 2 Phase 3 |
|
By Cancer Type |
Breast Cancer Lung Cancer Colorectal Cancer Other Cancers |
|
By Region |
North South East West |
Products
Key Target Audience – Organizations and Entities Who Can Benefit by Subscribing This Report:
Hospitals
Cancer Research Centers
Pharmaceutical Companies
Biotechnology Firms
Government Health Agencies
Medical Device Manufacturers
Health Insurance Companies
Diagnostic Laboratories
Clinical Trial Management Companies
Regulatory Authorities (BPOM, Ministry of Health and NADFC)
Time Period Captured in the Report:
Historical Period: 2018-2023
Base Year: 2023
Forecast Period: 2023-2028
Companies
Players Mentioned in the Report:Â
Kalbe Farma Tbk
Prodia Clinical Laboratory
Siloam International Hospitals
Mayapada Hospital Group
Mochtar Riady Comprehensive Cancer Centre (MRCCC)
Bio Farma
Kimia Farma Tbk
Tempo Scan Pacific Tbk
Darya-Varia Laboratoria Tbk
Soho Global Health
Enseval Putera Megatrading Tbk
Phapros Tbk
Dexa Medica
Industri Jamu Dan Farmasi Sido Muncul Tbk
Imunologi Fakultas Kedokteran Universitas Indonesia
Interbat
Kimia Farma Sungwun Pharmacopia
Novell Pharmaceutical Laboratories
Sanbe Farma
Pyridam Farma Tbk
Table of Contents
1. Indonesia Oncology Clinical Trials Market Overview
1.1 Indonesia Oncology Clinical Trials Market Taxonomy
2. Indonesia Oncology Clinical Trials Market Size (in USD Mn), 2018-2023
3. Indonesia Oncology Clinical Trials Market Analysis
3.1 Indonesia Oncology Clinical Trials Market Growth Drivers
3.2 Indonesia Oncology Clinical Trials Market Challenges and Issues
3.3 Indonesia Oncology Clinical Trials Market Trends and Development
3.4 Indonesia Oncology Clinical Trials Market Government Regulation
3.5 Indonesia Oncology Clinical Trials Market SWOT Analysis
3.6 Indonesia Oncology Clinical Trials Market Stake Ecosystem
3.7 Indonesia Oncology Clinical Trials Market Competition Ecosystem
4. Indonesia Oncology Clinical Trials Market Segmentation, 2023
4.1 Indonesia Oncology Clinical Trials Market Segmentation by Phase (in value %), 2023
4.2 Indonesia Oncology Clinical Trials Market Segmentation by Cancer Type (in value %), 2023
4.3 Indonesia Oncology Clinical Trials Market Segmentation by Region (in value %), 2023
5. Indonesia Oncology Clinical Trials Market Competition Benchmarking
5.1 Indonesia Oncology Clinical Trials Market Cross-Comparison (no. of employees, company overview, business strategy, USP, recent development, operational parameters, financial parameters and advanced analytics)
6. Indonesia Oncology Clinical Trials Future Market Size (in USD Mn), 2023-2028
7. Indonesia Oncology Clinical Trials Future Market Segmentation, 2028
7.1 Indonesia Oncology Clinical Trials Market Segmentation by Phase (in value %), 2028
7.2 Indonesia Oncology Clinical Trials Market Segmentation by Cancer Type (in value %), 2028
7.3 Indonesia Oncology Clinical Trials Market Segmentation by Region (in value %), 2028
8. Indonesia Oncology Clinical Trials Market Analysts’ Recommendations
8.1 Indonesia Oncology Clinical Trials Market TAM/SAM/SOM Analysis
8.2 Indonesia Oncology Clinical Trials Market Customer Cohort Analysis
8.3 Indonesia Oncology Clinical Trials Market Marketing Initiatives
8.4 Indonesia Oncology Clinical Trials Market White Space Opportunity Analysis
Research Methodology
Step: 1 Identifying Key Variables:
Ecosystem creation for all the major entities and referring to multiple secondary and proprietary databases to perform desk research around market to collate industry level information.Â
Step: 2 Market Building:
Collating statistics on Indonesia oncology clinical trials market over the years, penetration of marketplaces and service providers ratio to compute revenue generated for Indonesia oncology clinical trials market. We will also review service quality statistics to understand revenue generated which can ensure accuracy behind the data points shared.
Step: 3 Validating and Finalizing:
Building market hypothesis and conducting CATIs with industry experts belonging to different companies to validate statistics and seek operational and financial information from company representatives.Â
Step: 4 Research Output:
Our team will approach multiple oncology clinical trials institutes and understand nature of product segments and sales, consumer preference and other parameters, which will support us validate statistics derived through bottom to top approach from oncology clinical trials institutes.
Frequently Asked Questions
01 How big is Indonesia Oncology Clinical Trials Market?
The Indonesia oncology clinical trials market, valued at USD 80 Mn in 2023, is driven by rising incidence of cancer, increased funding for clinical trials, and the adoption of innovative technologies in cancer treatment.
02 What are the challenges in Indonesia Oncology Clinical Trials Market?
Challenges include patient recruitment and retention issues, lengthy and complex regulatory approval processes, limited access to advanced diagnostic and treatment technologies, and funding constraints for early-phase trials.
03 Who are the major players in Indonesia Oncology Clinical Trials Market?
Key players in the market include PT Kalbe Farma Tbk, Prodia Clinical Laboratory, Siloam International Hospitals, Mayapada Hospital Group, and Mochtar Riady Comprehensive Cancer Centre (MRCCC). These companies are pivotal in driving advancements in cancer research and treatment.
04 What are the growth drivers of the Indonesia Oncology Clinical Trials Market?
The market is propelled by the increasing cancer incidence, rising healthcare expenditure, expanding clinical trial networks, and government support for research and development. These factors are driving the demand for innovative cancer treatments and clinical trials.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















