
KSA Microfluidics Market Outlook to 2030
Region:Middle East
Author(s):Abhinav kumar
Product Code:KROD3888
December 2024
80
About the Report
KSA Microfluidics Market Overview
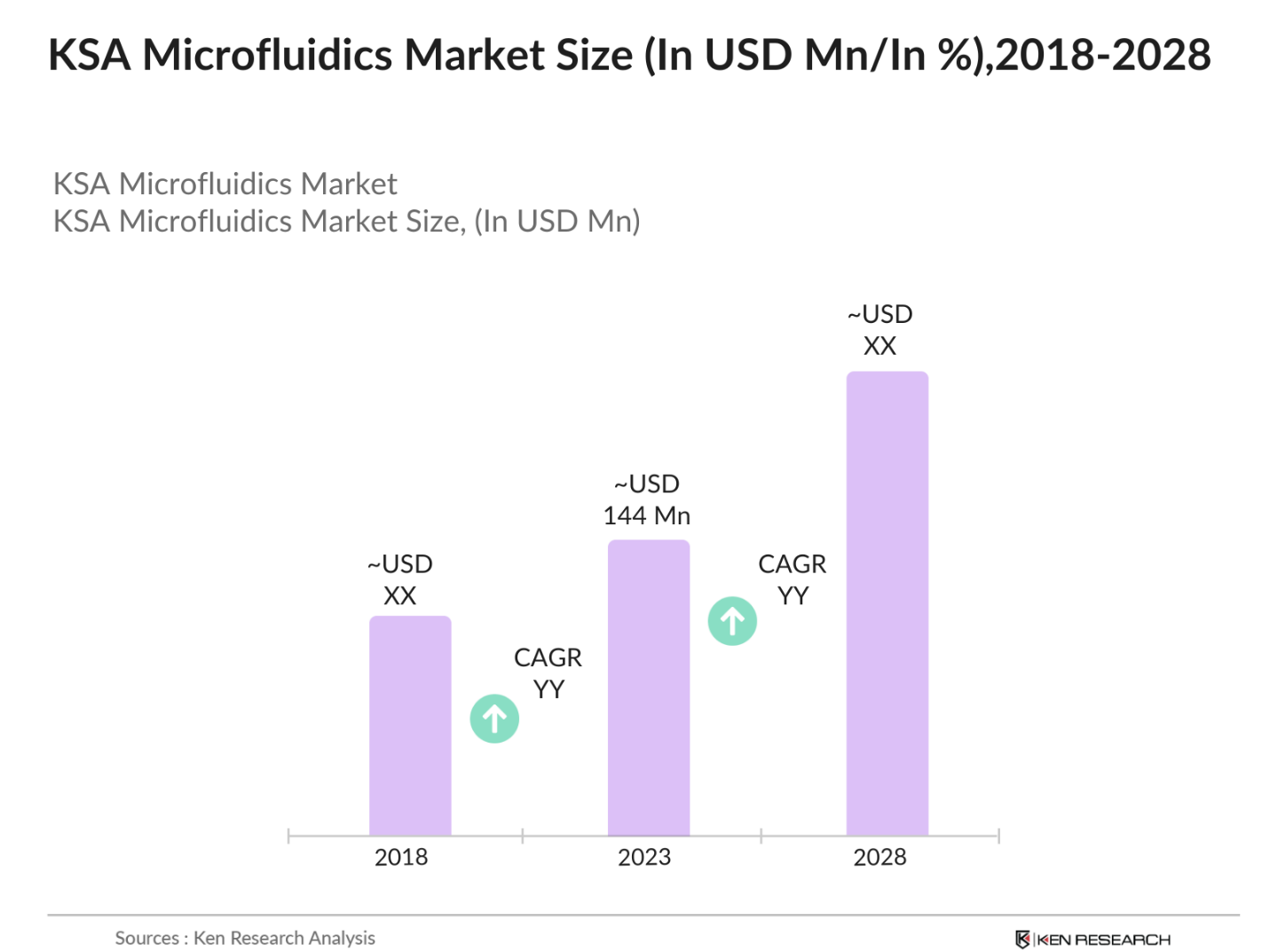
- The KSA microfluidics market is valued at USD 144 million, based on a five-year historical analysis, and is driven by the growing demand for advanced diagnostic tools in healthcare. With the increasing emphasis on point-of-care testing and personalized medicine, microfluidics technology plays a crucial role in providing precise and rapid testing solutions. Key drivers of this growth include the healthcare sectors demand for miniaturized systems that offer real-time diagnostic results, as well as advancements in lab-on-a-chip technologies that streamline processes in pharmaceutical and clinical applications. This demand is further fueled by the government's investments in biotechnology and healthcare infrastructure.
- The dominance of cities like Riyadh and Jeddah in the KSA microfluidics market is due to their advanced healthcare infrastructure and concentration of biotechnology companies. Riyadh, as the capital, attracts significant investment in medical technology research and development, making it a hub for innovation. Jeddah's strategic location as a commercial center enables it to host a growing number of pharmaceutical companies that contribute to the expansion of microfluidic technologies in the region. These cities have developed a robust ecosystem for high-tech healthcare solutions, leading to their market dominance.
- The National Air Quality Monitoring Program aims to install 500 monitoring units across the country by 2025. In 2023, 320 units were already operational, providing crucial data for policymakers to address air pollution. This program reflects the governments commitment to improving air quality and ensuring compliance with international environmental standards.
KSA Microfluidics Market Segmentation
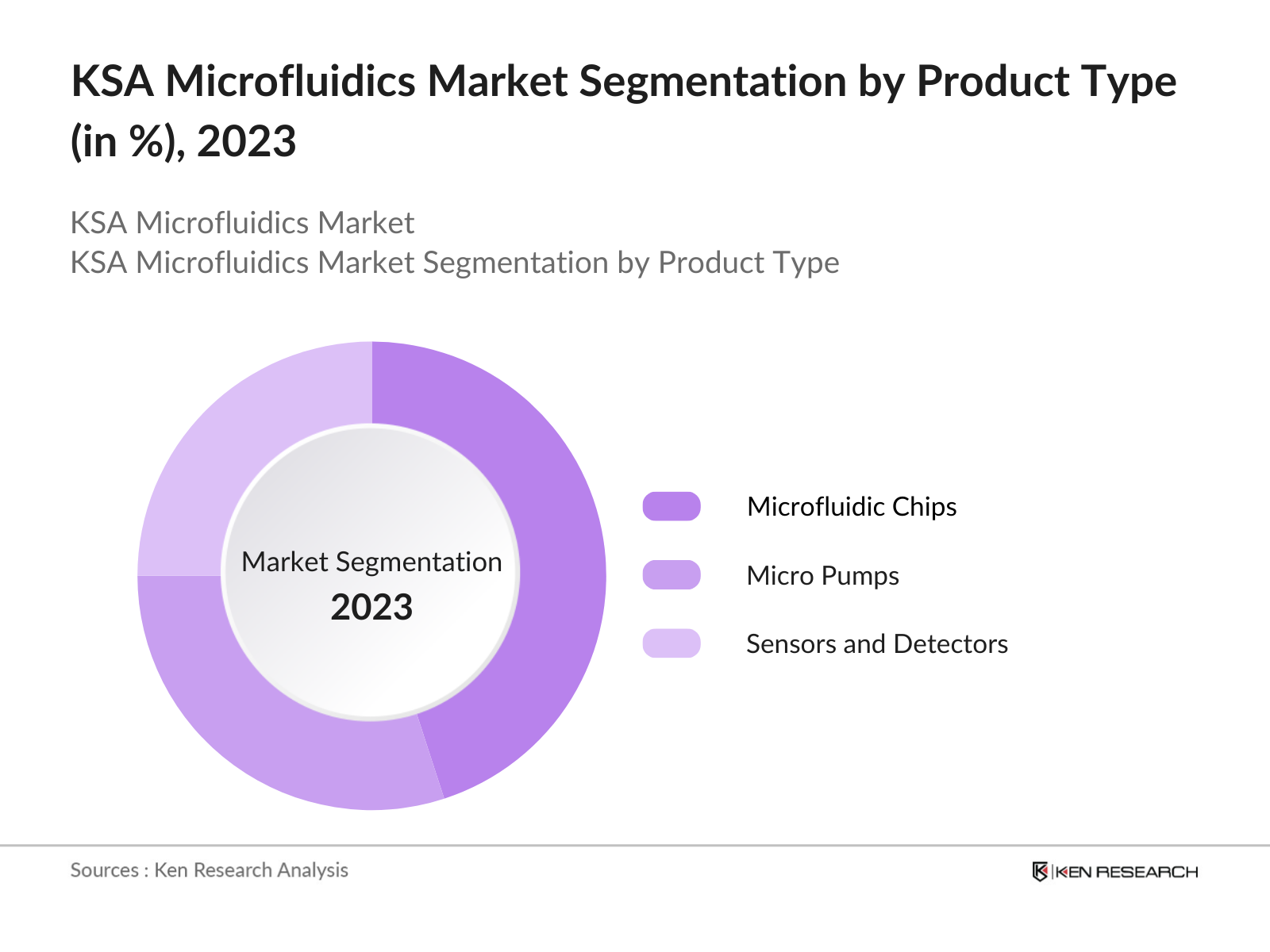
By Product Type: The KSA microfluidics market is segmented by product type into microfluidic chips, micro pumps, and sensors. Recently, microfluidic chips have dominated the market due to their wide applications in healthcare diagnostics, drug discovery, and biotechnological research. The ability of microfluidic chips to integrate multiple laboratory processes into a single chip has revolutionized the medical diagnostic field, particularly in point-of-care testing. Their ease of use, ability to process small volumes of fluids, and integration with other technologies, such as AI and IoT, have made microfluidic chips the go-to solution for many healthcare providers and researchers.
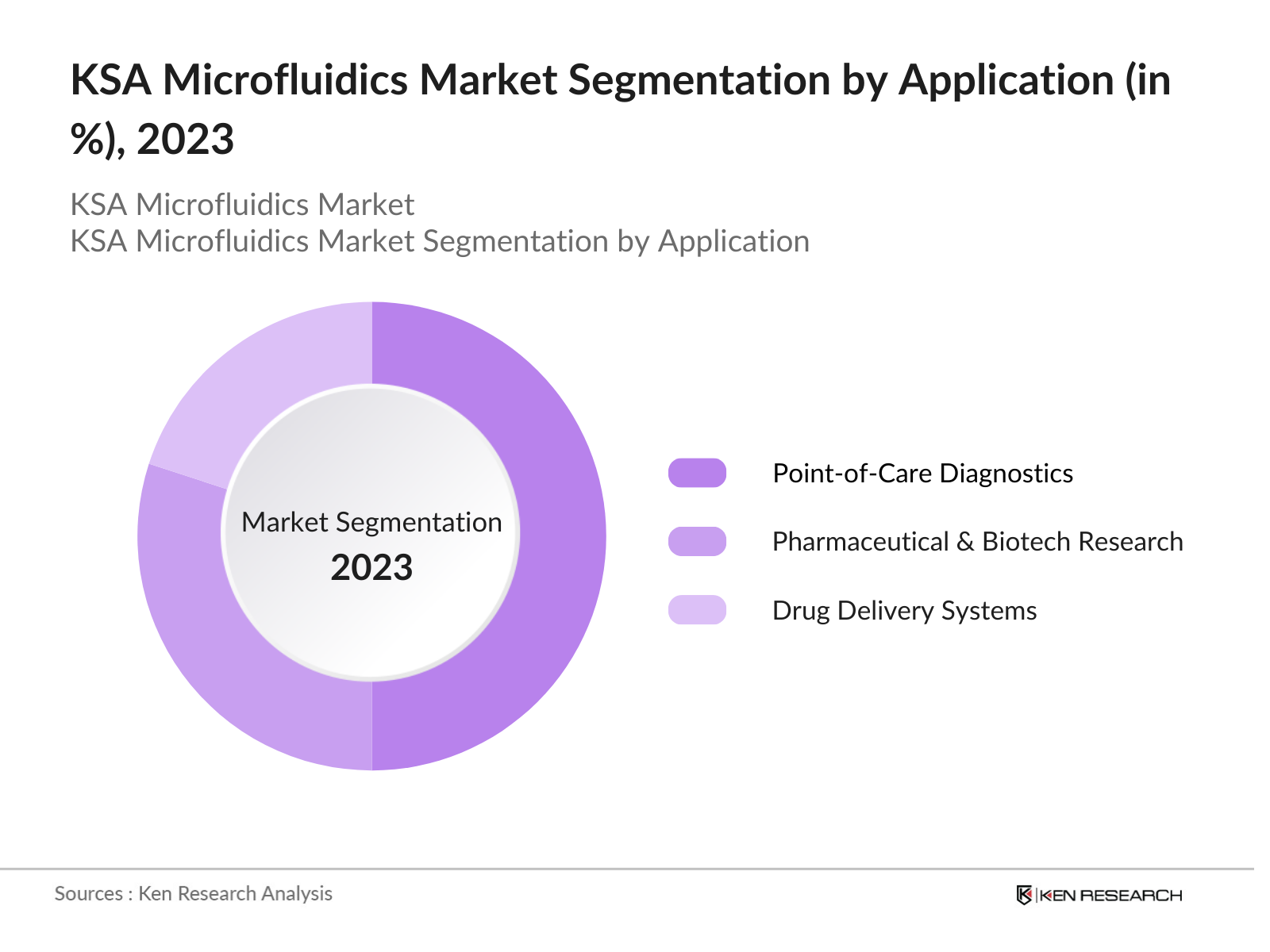
By Application: The KSA microfluidics market is also segmented by application into point-of-care diagnostics, pharmaceutical and biotechnology research, and drug delivery systems. Among these, point-of-care diagnostics holds a dominant market share due to the growing demand for real-time medical diagnostics in both clinical settings and remote locations. The ability of microfluidic devices to deliver quick and accurate results without the need for large laboratory equipment has significantly contributed to their adoption in the healthcare sector. This is particularly important in rural or underserved areas, where access to advanced healthcare infrastructure is limited.
KSA Microfluidics Market Competitive Landscape
The KSA microfluidics market is dominated by several key players, with both local and global companies playing a significant role. Companies such as Fluidigm Corporation and Bio-Rad Laboratories lead the market due to their extensive product portfolios and strong presence in the biotechnology and medical device industries. Local companies are also gaining traction due to government support and partnerships with global firms. This competitive landscape is characterized by significant investments in R&D and strategic collaborations aimed at driving innovation and expanding market share.
|
Company |
Established |
Headquarters |
R&D Investment |
Technological Innovations |
Product Portfolio |
Global Presence |
Manufacturing Capabilities |
Partnerships |
|
Fluidigm Corporation |
1999 |
USA |
High |
- |
- |
- |
- |
- |
|
Bio-Rad Laboratories |
1952 |
USA |
High |
- |
- |
- |
- |
- |
|
Agilent Technologies |
1999 |
USA |
Medium |
- |
- |
- |
- |
- |
|
Micronit Microtechnologies |
1999 |
Netherlands |
Medium |
- |
- |
- |
- |
- |
|
Elveflow |
2011 |
France |
Medium |
- |
- |
- |
- |
- |
KSA Microfluidics Industry Analysis
KSA Microfluidics Market Growth Drivers
Urbanization: The urban population in Indonesia has significantly increased, reaching 158 million in 2022, according to the World Bank. This rapid urbanization has intensified air quality issues, particularly in cities like Jakarta, where pollution levels regularly exceed safe thresholds. The expansion of urban centers drives the demand for air quality monitoring systems as the government aims to mitigate the health risks associated with poor air quality. With over 57% of the population living in urban areas, Indonesia is focusing on improving air quality through more sophisticated monitoring technologies.
Industrialization: Indonesia's industrial sector contributes about 39% of the GDP, and industries like mining, energy, and manufacturing are key drivers of air pollution. The need to monitor emissions from industrial operations has increased, especially in industrial zones like Bekasi and Tangerang. This rise in industrial activities has spurred demand for real-time air quality monitoring systems to comply with emission standards and avoid penalties under Indonesias environmental laws.
Government Regulations: In response to growing pollution concerns, Indonesias Ministry of Environment and Forestry has mandated the installation of air quality monitoring systems in high-risk pollution zones. As of 2024, the government has set emission reduction targets to control air pollutants, driving the demand for such monitoring systems across urban and industrial areas. The implementation of the National Air Quality Monitoring Program has seen the deployment of 300+ monitoring units nation wide.
KSA Microfluidics Market Challenges
- High Initial Costs: The cost of deploying air quality monitoring systems in Indonesia remains a significant challenge, with initial investments ranging between IDR 1.5 billion and IDR 2 billion per unit. These high costs deter smaller municipalities and industries from investing in the technology, especially in remote areas. Additionally, the lack of local manufacturing facilities for such systems further inflates prices due to import taxes and transportation costs.
- Technical Challenges: Maintaining the technical accuracy of air quality monitoring systems is a critical challenge in Indonesia, where environmental conditions like high humidity and heat can affect sensor performance. Frequent calibration and maintenance are required, and the country has limited technical expertise to address these issues effectively. The lack of skilled technicians also slows down the implementation of sophisticated monitoring technologies.
KSA Microfluidics Market Future Outlook
Over the next five years, the KSA microfluidics market is expected to experience substantial growth, driven by continuous advancements in medical technologies and increased demand for point-of-care diagnostic solutions. Government investments in biotechnology and healthcare infrastructure, combined with the rising prevalence of chronic diseases, will continue to propel market growth. Additionally, innovations in lab-on-a-chip and droplet-based microfluidics will further enhance the capabilities of healthcare providers to offer precise and efficient diagnostic and treatment solutions. The expansion of the healthcare sector and increasing collaborations with international biotechnology firms are also likely to play a significant role in the market's upward trajectory.
- Technological Advancements: Technological innovations, such as IoT-based sensors and cloud computing, are transforming air quality monitoring in Indonesia. These advancements enable real-time monitoring and data analysis, offering more precise measurements. In 2023, Indonesia invested IDR 5 billion into research on integrating these technologies into air quality management systems, highlighting the growing opportunity for the development of smarter, cost-effective solutions.
- Expansion into Rural Areas: With over 44% of the population living in rural areas, there is a growing need to expand air quality monitoring systems beyond urban centers. The government plans to install 200 new monitoring units in rural areas by the end of 2025, targeting regions affected by deforestation and agricultural emissions. This expansion provides an untapped market opportunity for companies in the air quality monitoring sector.
Scope of the Report
|
By Product Type |
Microfluidic Chips Micro Pumps Sensors |
|
By Application |
Point-of-Care Diagnostics Drug Delivery |
|
By Technology |
Continuous Flow Droplet-Based Digital |
|
By Material Type |
Polymers Glass Silicon |
|
By End-User |
Hospitals Research Labs Pharma Cos |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing to This Report:
Government and Regulatory Bodies (Saudi Food and Drug Authority, Ministry of Health)
Healthcare Industry
Biotechnology Companies
Pharmaceutical Companies
Diagnostic Laboratories Industry
Investors and Venture Capitalist Firms
Medical Devices Companies
Companies
Players Mentioned in the Report:
Fluidigm Corporation
Bio-Rad Laboratories Inc.
Agilent Technologies
Micronit Microtechnologies
Elveflow
Thermo Fisher Scientific Inc.
Danaher Corporation
PerkinElmer Inc.
Dolomite Microfluidics
Illumina, Inc.
Table of Contents
1. KSA Microfluidics Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. KSA Microfluidics Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. KSA Microfluidics Market Analysis
3.1. Growth Drivers
3.1.1. Expansion of Healthcare Sector
3.1.2. Adoption of Advanced Technologies
3.1.3. Government Support for R&D in Biotechnology
3.1.4. Rising Demand for Point-of-Care Testing
3.2. Market Challenges
3.2.1. Lack of Skilled Workforce in Advanced Manufacturing
3.2.2. Regulatory Challenges in Medical Devices
3.2.3. Limited Local Production Capabilities
3.3. Opportunities
3.3.1. Innovations in Lab-on-a-Chip Technologies
3.3.2. Increasing International Collaborations
3.3.3. Growing Demand for Personalized Medicine
3.4. Trends
3.4.1. Miniaturization of Microfluidic Devices
3.4.2. Integration with Artificial Intelligence and IoT
3.4.3. Expansion in Drug Discovery Applications
3.5. Government Regulations
3.5.1. Medical Device Regulatory Framework in KSA
3.5.2. Compliance with International Standards (ISO, FDA)
3.5.3. KSA Biotechnology Development Policies
3.6. SWOT Analysis
3.7. Stake Ecosystem (Suppliers, manufacturers, distributors, end-users)
3.8. Porters Five Forces Analysis (Microfluidics market)
3.9. Competition Ecosystem (Major players, local and international partnerships, distribution networks)
4. KSA Microfluidics Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Microfluidic Chips
4.1.2. Micro Pumps
4.1.3. Sensors and Detectors
4.2. By Application (In Value %)
4.2.1. Point-of-Care Diagnostics
4.2.2. Pharmaceutical & Biotechnology Research
4.2.3. Drug Delivery Systems
4.3. By Technology (In Value %)
4.3.1. Continuous Flow
4.3.2. Droplet-Based Microfluidics
4.3.3. Digital Microfluidics
4.4. By Material Type (In Value %)
4.4.1. Polymers
4.4.2. Glass
4.4.3. Silicon
4.5. By End-User (In Value %)
4.5.1. Hospitals and Clinics
4.5.2. Research Laboratories
4.5.3. Pharmaceutical Companies
5. KSA Microfluidics Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Fluidigm Corporation
5.1.2. Dolomite Microfluidics
5.1.3. PerkinElmer Inc.
5.1.4. Micronit Microtechnologies
5.1.5. Bio-Rad Laboratories Inc.
5.1.6. Agilent Technologies Inc.
5.1.7. Danaher Corporation
5.1.8. Thermo Fisher Scientific Inc.
5.1.9. Elveflow
5.1.10. RainDance Technologies
5.1.11. Qiagen N.V.
5.1.12. Parker Hannifin Corporation
5.1.13. Aline Inc.
5.1.14. Illumina, Inc.
5.1.15. Labcyte Inc.
5.2. Cross Comparison Parameters (Product Portfolio, R&D Expenditure, Global Presence, Collaborations, Manufacturing Capabilities, Technological Innovations, Market Penetration, Service Support)
5.3. Market Share Analysis (Percentage-wise breakdown by company)
5.4. Strategic Initiatives (Partnerships, joint ventures, strategic alliances)
5.5. Mergers and Acquisitions (Major deals in the last five years)
5.6. Investment Analysis (Capital inflow from private and public sources)
5.7. Government Funding and Grants (Local government initiatives)
5.8. Private Equity Investments (Recent investments in the microfluidics market)
6. KSA Microfluidics Market Regulatory Framework
6.1. Regulatory Bodies for Medical Devices
6.2. Compliance Requirements for Medical Device Manufacturing
6.3. Certification and Approval Processes (ISO, CE marking, FDA)
7. KSA Microfluidics Market Future Outlook (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. KSA Microfluidics Market Future Segmentation
8.1. By Product Type (In Value %)
8.2. By Application (In Value %)
8.3. By Technology (In Value %)
8.4. By Material Type (In Value %)
8.5. By End-User (In Value %)
9. KSA Microfluidics Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Innovation Pipeline Assessment
9.3. Product Launch Strategies
9.4. Strategic Market Expansion Recommendations
Disclaimer
Contact Us
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves mapping out the KSA microfluidics market ecosystem, identifying all major stakeholders. Extensive desk research is conducted using secondary sources such as government databases, healthcare reports, and proprietary databases to gather comprehensive market-level information.
Step 2: Market Analysis and Construction
In this phase, historical data is analyzed to assess the markets growth trajectory. Key metrics such as market penetration, device adoption rates, and revenue generation are evaluated to form a comprehensive market analysis. Furthermore, data on healthcare infrastructure and diagnostic capacities in KSA are analyzed to estimate future trends.
Step 3: Hypothesis Validation and Expert Consultation
Through consultations with industry experts and stakeholders, market hypotheses are validated. Experts provide insights into the operational and financial dynamics of the KSA microfluidics market, refining the accuracy of the data gathered.
Step 4: Research Synthesis and Final Output
The final phase consolidates the collected data, validated through a bottom-up approach, ensuring accuracy. This synthesis includes interviews with microfluidic device manufacturers and healthcare providers, helping to verify and complement the data collected from secondary sources.
Frequently Asked Questions
01. How big is the KSA Microfluidics Market?
The KSA microfluidics market is valued at USD 144 million, driven by advancements in point-of-care diagnostics and increased demand for lab-on-a-chip technologies.
02. What are the challenges in the KSA Microfluidics Market?
Challenges include high manufacturing costs, regulatory hurdles, and limited local production capabilities. Additionally, a shortage of skilled labor to handle advanced manufacturing processes is a growing concern.
03. Who are the major players in the KSA Microfluidics Market?
Key players include Fluidigm Corporation, Bio-Rad Laboratories, Agilent Technologies, and Micronit Microtechnologies, which dominate due to their strong R&D investments and extensive product portfolios.
04. What are the growth drivers of the KSA Microfluidics Market?
Growth drivers include the expansion of the healthcare sector, increased demand for rapid diagnostic solutions, and government investments in biotechnology. Technological innovations in microfluidic devices also propel market growth.
05. What applications dominate the KSA Microfluidics Market?
Point-of-care diagnostics is the dominant application in the KSA microfluidics market, as the demand for quick and accurate diagnostic tools in clinical settings and remote locations grows rapidly.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















