
North America CRO (Contract Research Organization) Market Outlook to 2030
Region:North America
Author(s):Naman Rohilla
Product Code:KROD4011
September 2024
92
About the Report
North America CRO Market Overview
- The North America CRO market is valued at USD 40 billion, driven by a five-year historical growth pattern focused on increasing pharmaceutical R&D activities and the rising demand for outsourcing services. CROs have become integral partners for biotechnology, pharmaceutical, and medical device companies as they look to streamline operations, reduce costs, and accelerate the time to market. Continuous advances in drug discovery technologies and the increasing complexity of clinical trials have pushed companies to rely heavily on CROs for full-service solutions.
- The United States holds the top position in terms of geographical dominance due to its well-established pharmaceutical sector, vast network of biotech startups, and robust healthcare infrastructure. Moreover, the U.S. government's supportive regulatory framework and extensive clinical trial networks make it an attractive destination for CRO services. Canada also plays a key role, focusing on innovation and partnerships between academic institutions and CROs. This dominance is underpinned by the sheer volume of clinical trials conducted in these regions.
- The FDA has implemented stringent guidelines to ensure the safety and efficacy of clinical trials, which CROs must follow closely. In 2023, the FDA released 23 new guidance documents aimed at improving clinical trial protocols, ensuring faster drug approvals. These guidelines provide a framework for CROs to navigate the complex approval processes, helping pharmaceutical companies accelerate drug development timelines. Compliance with FDA regulations is critical for CROs to maintain their credibility and secure contracts with large pharmaceutical firms.

North America CRO Market Segmentation
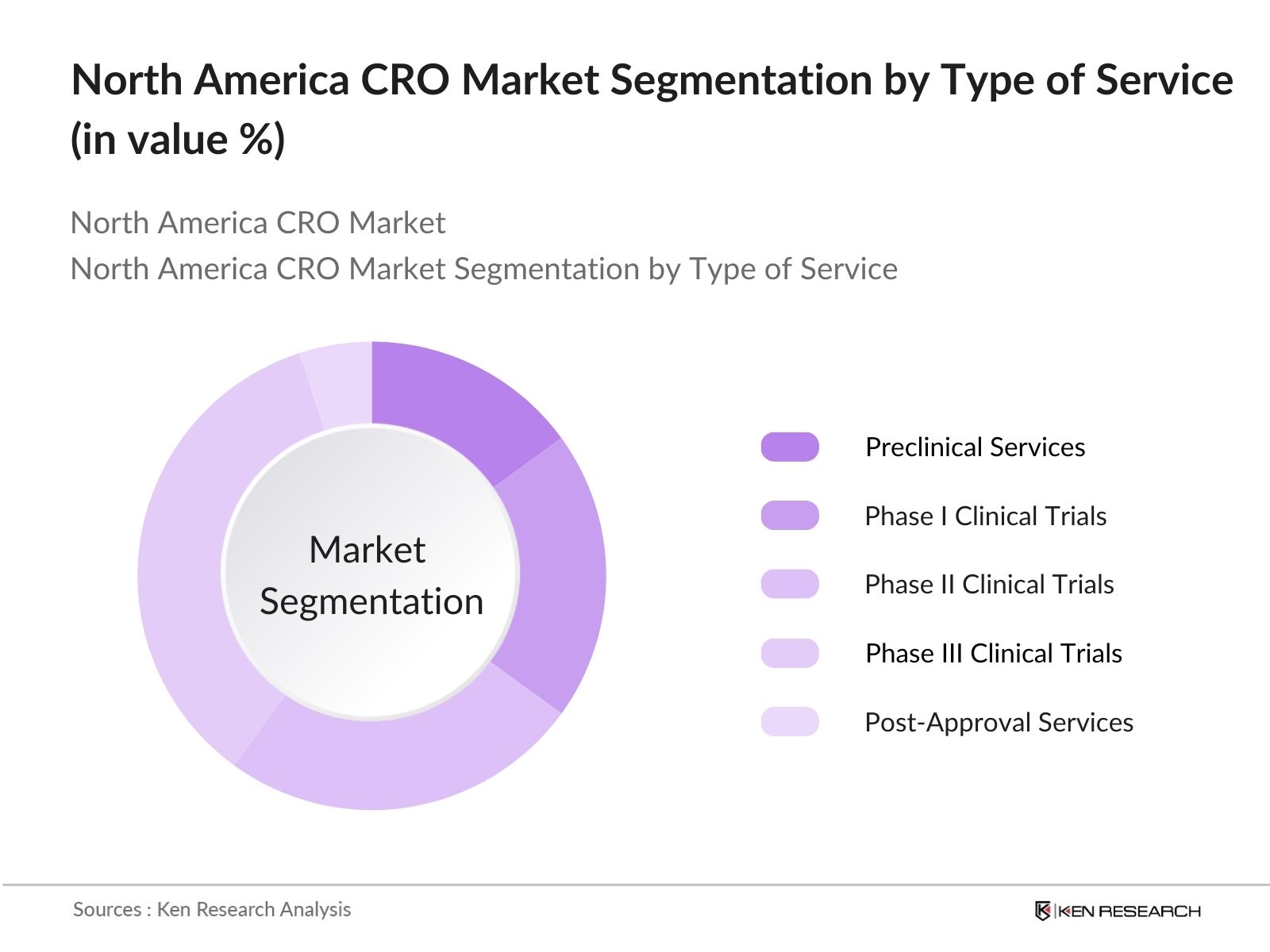
- By Type of Service: The North American CRO market is segmented by type of service into Preclinical Services, Phase I Clinical Trials, Phase II Clinical Trials, Phase III Clinical Trials, and Post-Approval Services. Among these, Phase III Clinical Trials hold the dominant share in 2023, accounting for approximately 35% of the market. This is attributed to the complexity and extensive duration of Phase III trials, which involve larger patient populations and are crucial for securing regulatory approvals. The investments required in this phase often prompt companies to outsource these services to CROs.
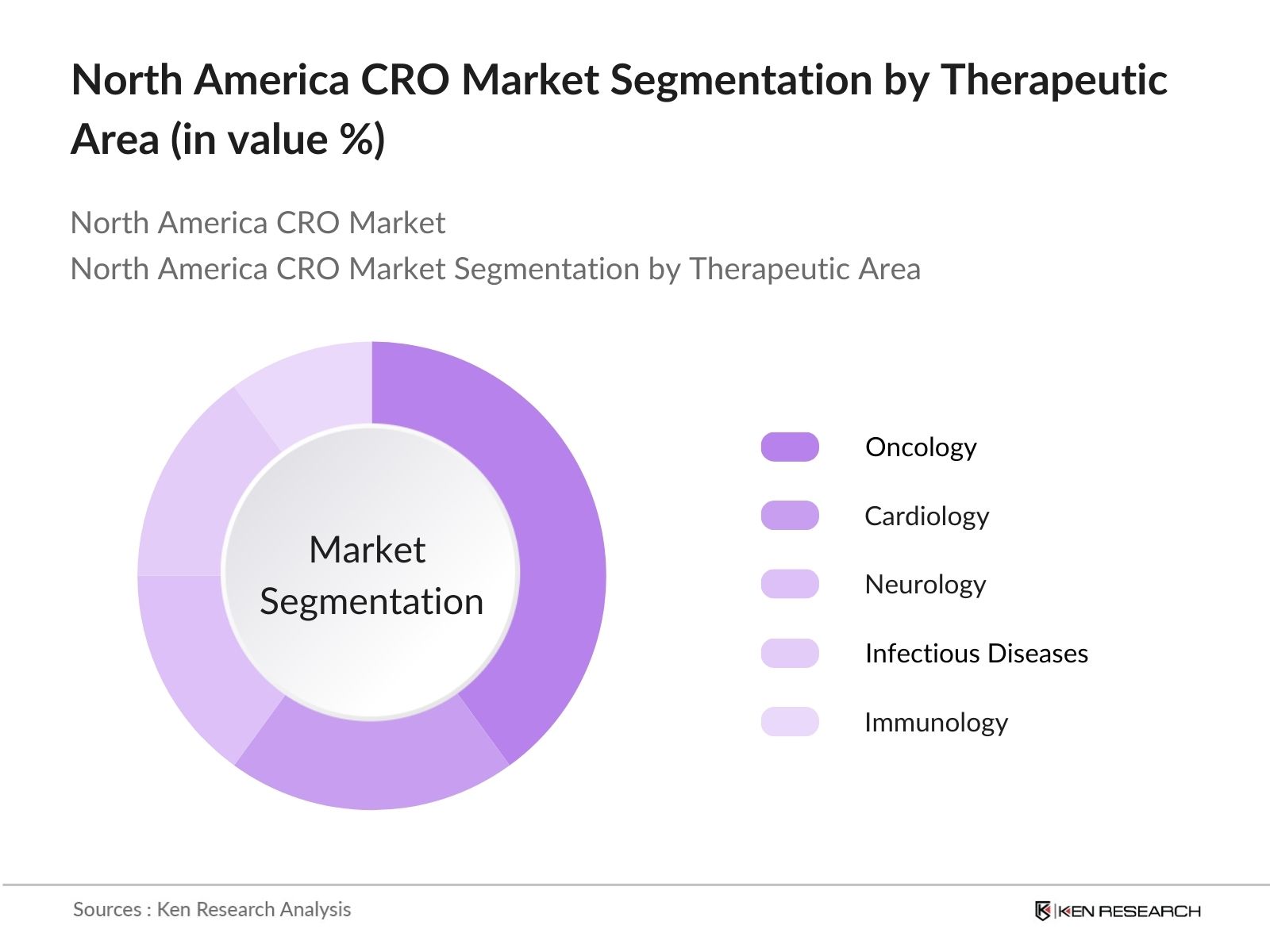
- By Therapeutic Area: The North America CRO market is also segmented by therapeutic area into Oncology, Cardiology, Neurology, Infectious Diseases, and Immunology. Oncology commands a dominant market share of 40% in 2023. This is driven by the rising number of cancer cases and the corresponding increase in oncology drug trials. Moreover, oncology trials are often complex, involving intricate protocols, which makes outsourcing to specialized CROs a preferred option for pharmaceutical companies.
North America CRO Market Competitive Landscape
The North America CRO market is dominated by a few key players that hold a substantial share of the market due to their comprehensive service offerings, global reach, and continuous investments in technology and innovation. These players include global giants such as IQVIA and Labcorp Drug Development, which have established themselves as leading providers of clinical trial services across various therapeutic areas.
| Company | Established | Headquarters | Clinical Trials Conducted | Revenue | Global Presence | Therapeutic Focus | Number of Employees | Strategic Partnerships | M&A Activity |
|---|---|---|---|---|---|---|---|---|---|
| IQVIA | 1982 | USA | - | - | - | - | - | - | - |
| Labcorp Drug Development | 1969 | USA | - | - | - | - | - | - | - |
| Syneos Health | 2017 | USA | - | - | - | - | - | - | - |
| PPD (Thermo Fisher) | 1985 | USA | - | - | - | - | - | - | - |
| ICON Plc | 1990 | Ireland | - | - | - | - | - | - | - |
North America CRO Market Analysis
North America CRO Market Growth Drivers
- Increased Outsourcing of R&D Activities: The pharmaceutical and biotechnology industries have been increasingly outsourcing R&D activities to contract research organizations (CROs), which has become a key driver of the North America CRO market. In 2023, the United States alone spent $92.6 billion on pharmaceutical R&D, according to the National Institutes of Health (NIH). This spending encourages companies to outsource R&D for cost-effectiveness and time-saving benefits, allowing them to focus on core competencies. The growing demand for external expertise in areas such as early-stage research and clinical trials is strengthening the CRO industry’s role in drug development.
- Rising Focus on Drug Discovery and Clinical Trials: North America has emerged as a global leader in drug discovery and clinical trials, particularly in the U.S. and Canada. The U.S. Food and Drug Administration (FDA) approved 52 novel drugs in 2023, highlighting the region's focus on rapid drug development. Additionally, North America is conducting over 30,000 clinical trials, according to the U.S. National Library of Medicine. This upsurge in drug discovery and trial activities fuels demand for CROs, which are often tasked with managing and overseeing these complex processes.
- Regulatory Support for Accelerated Approvals: The regulatory framework in North America, particularly with initiatives like the FDA's accelerated approval pathway, has been instrumental in speeding up drug approvals. In 2023, 13 therapies received accelerated approval from the FDA, compared to 10 in 2022, according to the FDA Annual Report. This regulatory support benefits CROs, as companies increasingly rely on them to navigate the complexities of regulatory submissions and trial protocols, ensuring faster market entry for novel drugs.
North America CRO Market Challenges
- High Competition and Pricing Pressure: The North American CRO market faces high competition, with over 1,000 active CROs in the region as of 2023, according to the U.S. Bureau of Labor Statistics. This competitive landscape exerts pricing pressure on both large and small CROs, forcing them to offer competitive pricing while maintaining service quality. The abundance of CRO options allows pharmaceutical companies to negotiate lower fees, leading to reduced margins for CRO providers.
- Regulatory Complexities: Navigating the complex regulatory requirements across multiple jurisdictions, particularly for global trials, is a major challenge. North American CROs must comply with various regulations, including FDA, Health Canada, and European Medicines Agency guidelines. In 2023, the FDA issued 47 clinical trial-related warning letters, reflecting the stringent regulatory environment. This complexity increases the workload for CROs and raises the risk of compliance issues, which can delay trial timelines and add additional costs.
North America CRO Market Future Outlook
Over the next five years, the North America CRO market is expected to show substantial growth driven by increasing demand for outsourcing clinical trials, rapid advances in technology, and the growing complexity of drug development. The rise of precision medicine, digital health solutions, and the application of artificial intelligence in drug discovery will further enhance the role of CROs in clinical research. The expansion of biotechnology firms and the increasing number of small- to mid-size companies entering the pharmaceutical sector will also fuel demand for CRO services.
North America CRO Market Opportunities
- Expanding Scope in Precision Medicine: Precision medicine has gained traction in North America, with over $1 billion in funding allocated to precision medicine initiatives in 2023, according to the National Institutes of Health (NIH). This approach tailors medical treatments to individual patient characteristics, requiring specialized clinical trials and data analytics, areas where CROs play a critical role. The growing demand for personalized treatments creates a new avenue for CROs to expand their services, particularly in genomics, biomarker research, and patient stratification.
- Increased Demand for Full-Service CROs: Pharmaceutical and biotechnology companies are increasingly seeking full-service CROs that can manage the entire drug development lifecycle, from early-stage research to post-marketing surveillance. In 2023, 70% of clinical trials in North America were outsourced to full-service CROs, according to the U.S. Department of Health and Human Services. This trend reflects the industry's growing preference for integrated solutions, offering growth opportunities for full-service providers capable of handling end-to-end clinical research services.
Scope of the Report
| By Type of Service |
Preclinical Services Phase I Clinical Trials Phase II Clinical Trials Phase III Clinical Trials Post-Approval Services |
| By Therapeutic Area |
Oncology Cardiology Neurology Infectious Diseases Immunology |
| By End-User |
Pharmaceutical Companies Biotechnology Companies Academic Institutes Medical Device Companies Government Institutions |
| By Region |
United States Canada Mexico |
Products
Key Target Audience
Pharmaceutical Companies
Biotechnology Firms
Medical Device Manufacturers
Government Agencies (FDA, Health Canada)
Hospitals and Research Institutes
Banks and Financial Institutions
Investment and Venture Capitalist Firms
Regulatory Bodies (IRB, Clinical Trial Compliance)
Contract Manufacturing Organizations (CMOs)
Companies
North America CRO Market Major Players
IQVIA
Labcorp Drug Development
Syneos Health
PPD (Thermo Fisher)
ICON Plc
Medpace
Charles River Laboratories
Parexel
PRA Health Sciences
KCR CRO
Worldwide Clinical Trials
Novotech
Covance
Wuxi AppTec
Pharmaceutical Product Development (PPD)
Table of Contents
1. North America CRO Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy
1.3 Market Growth Rate
1.4 Market Segmentation Overview
2. North America CRO Market Size (In USD Bn)
2.1 Historical Market Size
2.2 Year-On-Year Growth Analysis
2.3 Key Market Developments and Milestones
3. North America CRO Market Analysis
3.1 Growth Drivers
3.1.1 Increased Outsourcing of R&D Activities
3.1.2 Rising Focus on Drug Discovery and Clinical Trials
3.1.3 Technological Advancements in Data Analytics
3.1.4 Regulatory Support for Accelerated Approvals
3.2 Market Challenges
3.2.1 High Competition and Pricing Pressure
3.2.2 Regulatory Complexities (Global Regulatory Compliance)
3.2.3 Skilled Workforce Shortage
3.2.4 Complexities in Trial Management
3.3 Opportunities
3.3.1 Expanding Scope in Precision Medicine
3.3.2 Increased Demand for Full-Service CROs
3.3.3 Growth in Biotechnology and Biosimilar Markets
3.4 Trends
3.4.1 Increasing Use of AI in Clinical Research
3.4.2 Decentralized Clinical Trials (DCT)
3.4.3 Data Integration and E-Clinical Platforms
3.4.4 Focus on Real-World Evidence (RWE)
3.5 Government Regulations
3.5.1 FDA Guidelines and Approval Framework
3.5.2 Data Privacy Regulations (HIPAA, GDPR)
3.5.3 Good Clinical Practice (GCP) Compliance
3.5.4 Clinical Trial Transparency Initiatives
3.6 SWOT Analysis
3.7 Stakeholder Ecosystem
3.8 Porter’s Five Forces Analysis
3.9 Competition Ecosystem
4. North America CRO Market Segmentation
4.1 By Type of Service (In Value %)
4.1.1 Preclinical Services
4.1.2 Phase I Clinical Trials
4.1.3 Phase II Clinical Trials
4.1.4 Phase III Clinical Trials
4.1.5 Post-Approval Services
4.2 By Therapeutic Area (In Value %)
4.2.1 Oncology
4.2.2 Cardiology
4.2.3 Neurology
4.2.4 Infectious Diseases
4.2.5 Immunology
4.3 By End-User (In Value %)
4.3.1 Pharmaceutical Companies
4.3.2 Biotechnology Companies
4.3.3 Academic Institutes
4.3.4 Medical Device Companies
4.3.5 Government Institutions
4.4 By Region (In Value %)
4.4.1 United States
4.4.2 Canada
4.4.3 Mexico
5. North America CRO Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1 IQVIA
5.1.2 Labcorp Drug Development
5.1.3 Syneos Health
5.1.4 PPD (Thermo Fisher)
5.1.5 ICON Plc
5.1.6 Medpace
5.1.7 Parexel
5.1.8 PRA Health Sciences
5.1.9 Charles River Laboratories
5.1.10 Covance
5.1.11 KCR CRO
5.1.12 Worldwide Clinical Trials
5.1.13 Pharmaceutical Product Development (PPD)
5.1.14 Novotech
5.1.15 Wuxi AppTec
5.2 Cross Comparison Parameters (Number of Clinical Trials Conducted, Revenue, Global Presence, Therapeutic Area Focus, Number of Employees, Year of Inception, Strategic Partnerships, M&A Activity)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers and Acquisitions
5.6 Investment Analysis
5.7 Government Support and Grants
5.8 Venture Capital Funding
6. North America CRO Market Regulatory Framework
6.1 Clinical Trial Protocol Compliance
6.2 Institutional Review Board (IRB) Approvals
6.3 Patient Safety and Data Integrity Standards
6.4 Ethical Considerations and Certifications
7. North America CRO Future Market Size (In USD Bn)
7.1 Future Market Size Projections
7.2 Key Factors Driving Future Market Growth
8. North America CRO Future Market Segmentation
8.1 By Type of Service (In Value %)
8.2 By Therapeutic Area (In Value %)
8.3 By End-User (In Value %)
8.4 By Region (In Value %)
9. North America CRO Market Analysts’ Recommendations
9.1 TAM/SAM/SOM Analysis
9.2 Customer Cohort Analysis
9.3 Strategic Growth Recommendations
9.4 White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
This phase involves mapping out the North America CRO market’s stakeholder ecosystem, using desk research and proprietary databases to gather insights on major players, service offerings, and revenue models.
Step 2: Market Analysis and Construction
We compile and analyze historical data on market penetration, trial success rates, and associated revenue streams, ensuring accurate estimates by cross-referencing service provider performance metrics.
Step 3: Hypothesis Validation and Expert Consultation
Our market hypotheses are validated through interviews with industry experts, offering direct insights into CRO operational dynamics and financial benchmarks.
Step 4: Research Synthesis and Final Output
Final research outputs are synthesized through detailed engagement with CRO service providers, confirming market trends and validating revenue figures using both top-down and bottom-up approaches.
Frequently Asked Questions
01. How big is the North America CRO market?
The North America CRO market was valued at USD 40 billion, driven by increased outsourcing of clinical trials and the growing complexity of drug development.
02. What are the challenges in the North America CRO market?
Challenges in the North America CRO market include regulatory hurdles, high competition among CROs, and the need for specialized services. The complexity of clinical trials also adds pressure on service providers.
03. Who are the major players in the North America CRO market?
Key players in the North America CRO market include IQVIA, Labcorp Drug Development, Syneos Health, PPD (Thermo Fisher), and ICON Plc. These companies dominate due to their extensive service portfolios and global reach.
04. What are the growth drivers of the North America CRO market?
North America CRO Market Growth is propelled by rising demand for clinical outsourcing, advancements in biotechnology, and increasing investments in drug development.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















