
North America Urolithiasis Management Devices Market Outlook to 2030
Region:North America
Author(s):Shubham Kashyap
Product Code:KROD11295
December 2024
91
About the Report
North America Urolithiasis Management Devices Market Overview
- The North America urolithiasis management devices market is rapidly evolving, reaching a market size of USD 385 Mn, driven by rising cases of kidney stones and advancements in minimally invasive treatment options. The increasing prevalence of lifestyle diseases like obesity, diabetes, and hypertension is significantly contributing to the rise in kidney stone incidences. The market for urolithiasis management devices is primarily supported by innovations in lithotripsy devices, ureteroscopes, and nephroscopes, which are transforming the way kidney stones are treated, particularly in outpatient settings.
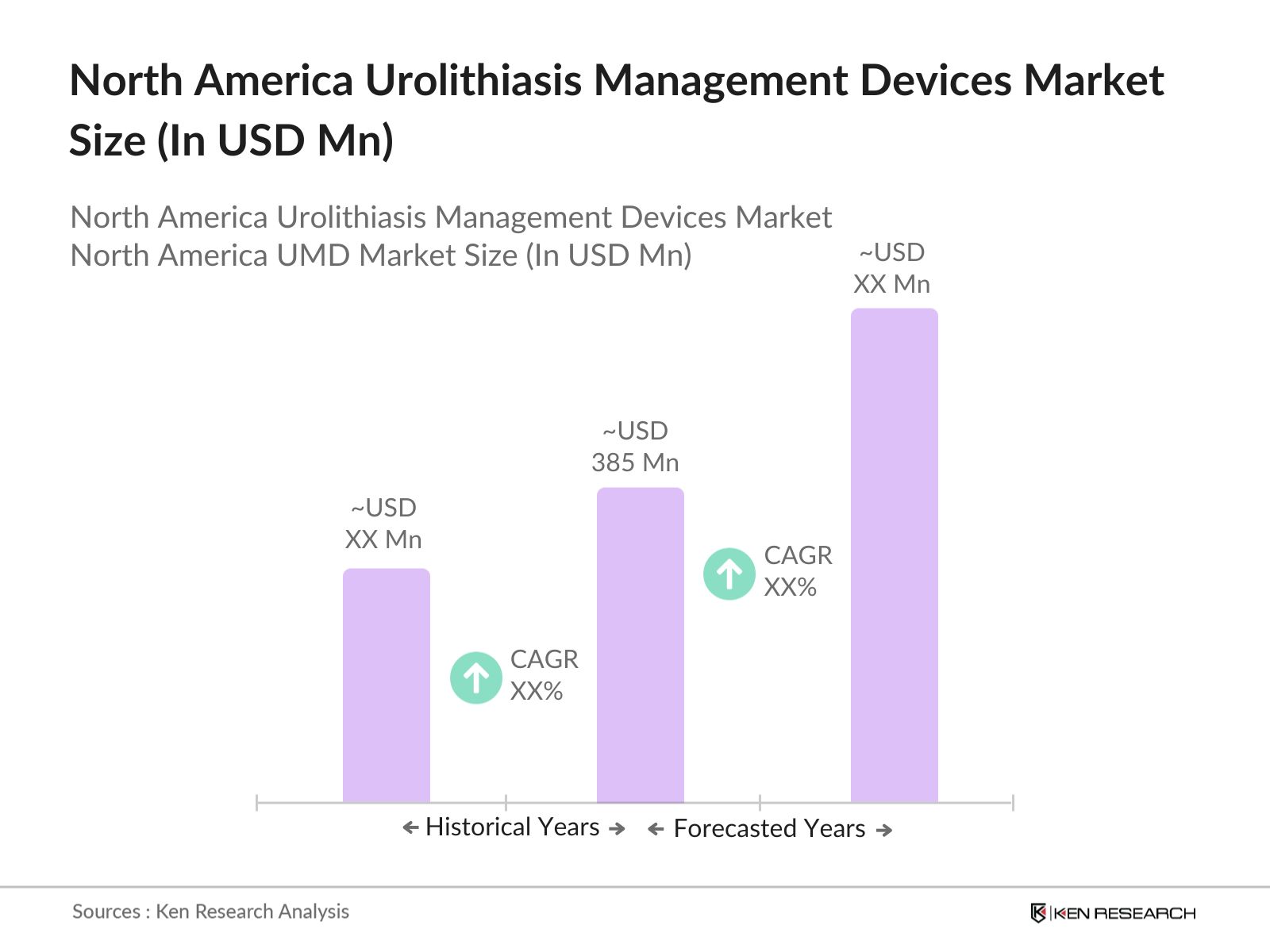
- The U.S. is leading the region in terms of adoption of advanced urolithiasis management technologies, with numerous healthcare institutions investing in cutting-edge devices to improve patient outcomes. Canada is also witnessing an upsurge in demand for these devices, driven by the increasing need for non-invasive treatments for kidney stones. Both countries are expected to see continued growth in the urolithiasis device sector as healthcare systems aim to reduce hospital stays and improve patient recovery times.
- Government healthcare initiatives, particularly in the U.S., aimed at improving access to advanced medical technologies, are further boosting the urolithiasis management devices market. Programs like the Affordable Care Act (ACA) have increased the number of patients seeking advanced treatments for kidney stones, particularly in outpatient and ambulatory settings. Additionally, the growing elderly population in North America, who are more prone to kidney stone formation, is further driving the market growth.
North America Urolithiasis Management Devices Market Segmentation
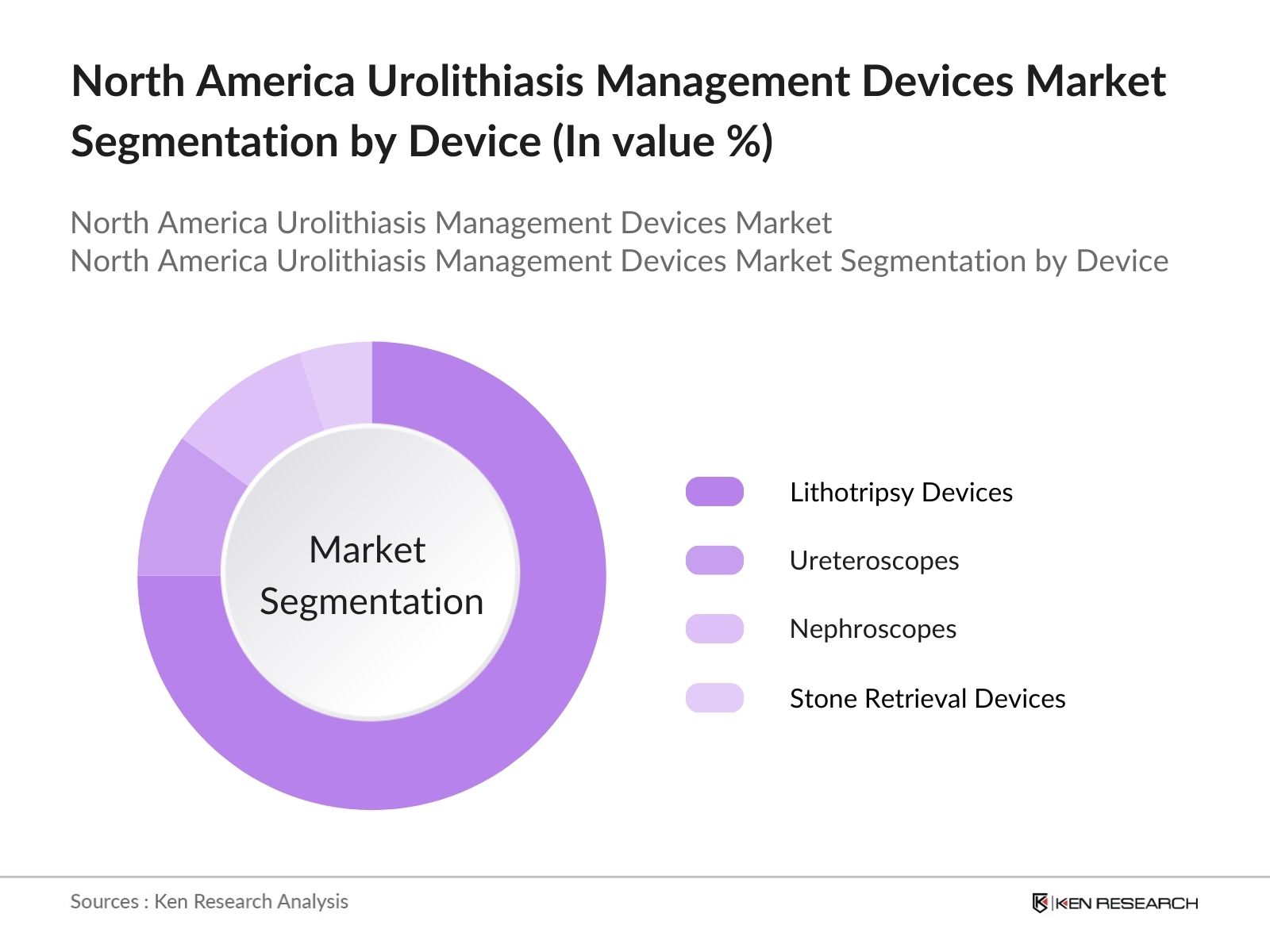
- By Device: The market in North America is segmented by device type into lithotripsy devices, ureteroscopes, nephroscopes, and stone retrieval devices. Lithotripsy devices dominate the market due to their non-invasive nature and effectiveness in breaking down kidney stones. Ureteroscopes are also gaining popularity, particularly for the treatment of stones located in the lower urinary tract. Nephroscopes are widely used in percutaneous nephrolithotomy procedures, especially for larger stones that cannot be treated with lithotripsy. Other devices, including stone retrieval baskets and guidewires, complement these primary treatment tools and are seeing increasing usage.
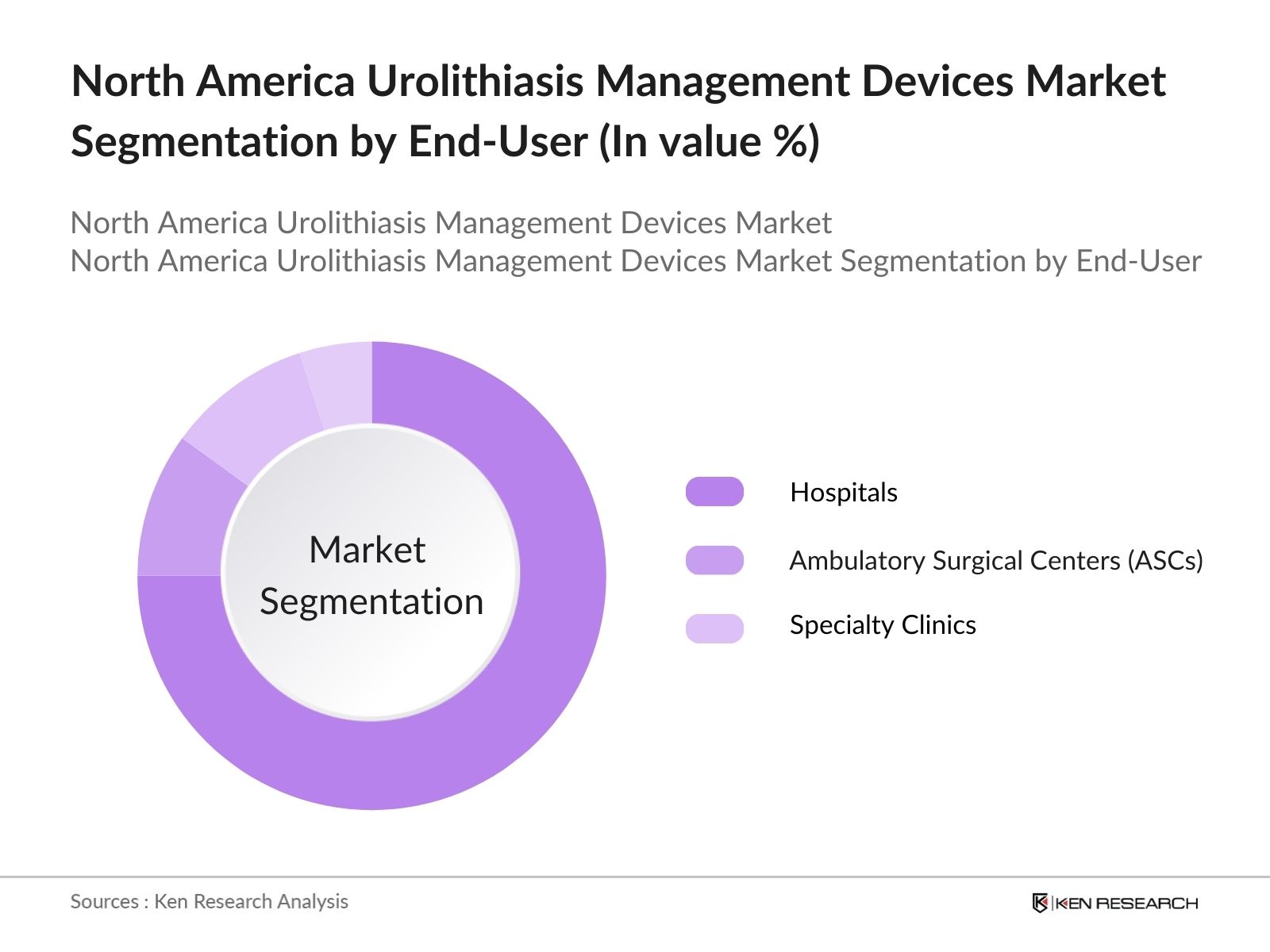
- By End-User: The market is segmented by end-user into hospitals, ambulatory surgical centers (ASCs), and specialty clinics. Hospitals account for the largest market share due to the availability of advanced equipment and expertise required for complex procedures. However, the growing preference for minimally invasive treatments is driving the demand for urolithiasis management devices in ASCs and specialty clinics, where patients can receive treatment in an outpatient setting. These facilities offer quicker recovery times and lower treatment costs, making them attractive options for both patients and healthcare providers.
North America Urolithiasis Management Devices Market Competitive Landscape
The North America urolithiasis management devices market is highly competitive, with both global and regional players introducing new technologies and expanding their product portfolios. Key players such as Boston Scientific, Olympus Corporation, and Cook Medical are at the forefront of innovation in this market. These companies are focusing on improving the efficacy of their devices while minimizing patient discomfort and recovery times.
|
Company Name |
Establishment Year |
Headquarters |
No. of Employees |
R&D Investment |
Product Portfolio Depth |
Global Reach |
Recent Partnerships |
Device Specialization |
|
Boston Scientific |
1979 |
Marlborough, MA, USA |
||||||
|
Cook Medical |
1963 |
Bloomington, IN, USA |
||||||
|
Olympus Corporation |
1919 |
Tokyo, Japan |
||||||
|
Dornier MedTech |
1983 |
Munich, Germany |
||||||
|
Karl Storz |
1945 |
Tuttlingen, Germany |
North America Urolithiasis Management Devices Industry Analysis
Growth Drivers:
- Rising Prevalence of Kidney Stones: The prevalence of kidney stones in North America has risen sharply due to dietary habits, obesity, and lack of physical activity. According to the U.S. National Institutes of Health, over 600,000 cases of kidney stone disease are diagnosed annually in the U.S. The Canadian Institute for Health Information also reports a substantial increase in kidney stone-related hospitalizations from 2020 to 2023, primarily in adults over the age of 50. This increase has driven demand for urolithiasis management devices, pushing healthcare facilities to adopt advanced treatment solutions to manage the growing patient base effectively.
- Technological Advancements in Minimally Invasive Devices: Technological innovations such as laser lithotripsy and flexible ureteroscopes have made minimally invasive treatments more effective, reducing recovery times and improving patient outcomes. In 2023, over 450,000 laser lithotripsy procedures were performed in North America, up from 370,000 in 2021, as reported by the American Urological Association. These procedures are favored because they eliminate the need for open surgeries, making them a preferred option for both patients and healthcare providers. Such innovations are significantly contributing to the increased adoption of advanced urolithiasis management devices.
- Growing Adoption of Minimally Invasive Procedures: Outpatient procedures for urolithiasis treatments have seen a sharp rise in North America, reducing hospital stays and costs for patients. According to the U.S. Centers for Medicare & Medicaid Services, majority of urolithiasis treatments, including lithotripsy and ureteroscopy, were performed in outpatient settings in 2023. This trend is driven by advancements in minimally invasive technologies, allowing patients to return home the same day, cutting down on healthcare costs and boosting demand for outpatient urology equipment.
Market Challenges:
- High Costs of Advanced Urolithiasis Devices: The cost of acquiring and maintaining advanced urolithiasis management devices, such as laser lithotripters and flexible ureteroscopes, remains a challenge. These devices represent a substantial financial investment for hospitals and clinics, particularly smaller facilities and those in rural areas. High upfront costs can act as a barrier to the adoption of newer, more efficient treatment technologies. This financial burden can hinder the market's growth, even though demand for advanced treatment options continues to rise.
- Technical Challenges in Device Operation: Operating advanced urolithiasis management devices, such as robotic ureteroscopes and lithotripters, presents technical challenges that require specialized training. Many urologists may not have received adequate training on the latest robotic-assisted technologies, limiting the adoption of these devices in clinical practice. Additionally, the need for continuous upgrades and regular device calibration adds complexity to their operation, making it harder for healthcare providers to fully integrate these advanced solutions into their practices.
North America Urolithiasis Management Devices Market Future Outlook
The North America urolithiasis management devices market is expected to experience sustained growth, driven by technological advancements, increasing patient awareness, and the rising demand for minimally invasive treatments. The market will likely see further developments in lithotripsy technology, as well as greater adoption of ureteroscopes and nephroscopes across the region.
Future Market Opportunities
- Rising Demand for Non-Invasive Lithotripsy Procedures: Non-invasive lithotripsy, such as extracorporeal shock wave lithotripsy (ESWL), is gaining popularity due to its effectiveness in treating kidney stones without surgery. In 2023, over thousands of ESWL procedures were performed in the U.S., as reported by the National Kidney Foundation. The growing preference for non-invasive procedures, which reduce recovery time and complications, is driving the demand for advanced lithotripsy devices across North America. This shift toward non-invasive treatment options presents significant opportunities for manufacturers to introduce cutting-edge devices.
- Emergence of Disposable Ureteroscopes and Single-Use Devices: The growing awareness of infection control and cost management in healthcare is driving demand for disposable ureteroscopes and other single-use urology devices. According to a 2022 report by the Centers for Disease Control and Prevention, a major portion of hospital-acquired infections in urology departments are linked to reusable devices. The adoption of disposable devices is expected to reduce infection risks and procedural costs, creating a lucrative market opportunity for manufacturers to develop and supply these products.
Scope of the Report
|
By Device Type |
Lithotripsy Devices Ureteroscopes Nephroscopes Stone Retrieval Devices |
|
By Technology |
Extracorporeal Shock Wave Lithotripsy (ESWL) Laser Lithotripsy Ultrasound Lithotripsy Percutaneous Nephrolithotomy (PCNL) |
|
By End-User |
Hospitals Ambulatory Surgical Centers (ASCs) Specialty Urology Clinics |
|
By Treatment Type |
Minimally Invasive Treatments Non-Invasive Treatments Invasive Treatments |
|
By Region |
United States Canada Mexico |
Products
Key Target Audience
Medical Device Manufacturers
Hospitals and Healthcare Providers
Ambulatory Surgical Centers (ASCs)
Specialty Urology Clinics
Investors and Venture Capitalist Firms
Government and Regulatory Bodies (U.S. FDA, Health Canada)
Urology Specialists and Surgeons
Banks and Financial Institutes
Medical Device Distributors
Banks and Financial Institutions
Companies
Major Players Mentioned in the Report
Boston Scientific
Cook Medical
Olympus Corporation
Dornier MedTech
Karl Storz
Medtronic
Siemens Healthineers
Stryker Corporation
Richard Wolf GmbH
EDAP TMS
Coloplast Group
Lumenis Ltd.
Bard Medical (BD)
STORZ MEDICAL AG
Quanta System
Table of Contents
1. North America Urolithiasis Management Devices Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. North America Urolithiasis Management Devices Market Size (In USD Mn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. North America Urolithiasis Management Devices Market Analysis
3.1. Growth Drivers
3.1.1. Increasing Prevalence of Kidney Stones
3.1.2. Technological Advancements in Minimally Invasive Devices
3.1.3. Growing Adoption of Outpatient Procedures
3.1.4. Government Healthcare Initiatives and Reimbursement Policies
3.2. Market Challenges
3.2.1. High Costs of Advanced Urolithiasis Devices
3.2.2. Technical Barriers in Device Operations
3.2.3. Limited Access to Treatment in Rural Areas
3.2.4. Stringent Regulatory Approval Processes
3.3. Opportunities
3.3.1. Rising Demand for Non-Invasive Lithotripsy Procedures
3.3.2. Emergence of Disposable Ureteroscopes and Single-Use Devices
3.3.3. Untapped Markets in Ambulatory Surgical Centers (ASCs)
3.3.4. Advancements in Robotics for Urology Procedures
3.4. Trends
3.4.1. Adoption of Laser Technology in Stone Fragmentation
3.4.2. Integration of AI and Imaging Technologies for Stone Detection
3.4.3. Growth in Endoscopic Lithotripsy Procedures
3.4.4. Development of Cost-Effective Urology Devices
3.5. Government Regulations
3.5.1. FDA Approvals and Regulatory Framework
3.5.2. U.S. Medicare and Medicaid Reimbursement Policies
3.5.3. Canadian Healthcare Regulations on Urology Devices
3.5.4. Occupational Safety and Handling Protocols
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem
3.8. Porters Five Forces Analysis
3.9. Competition Ecosystem
4. North America Urolithiasis Management Devices Market Segmentation
4.1. By Device Type (In Value %) 4.1.1. Lithotripsy Devices
4.1.2. Ureteroscopes
4.1.3. Nephroscopes
4.1.4. Stone Retrieval Devices
4.2. By Technology (In Value %) 4.2.1. Extracorporeal Shock Wave Lithotripsy (ESWL)
4.2.2. Laser Lithotripsy
4.2.3. Ultrasound Lithotripsy
4.2.4. Percutaneous Nephrolithotomy (PCNL)
4.3. By End-User (In Value %) 4.3.1. Hospitals
4.3.2. Ambulatory Surgical Centers (ASCs)
4.3.3. Specialty Urology Clinics
4.4. By Treatment Type (In Value %) 4.4.1. Minimally Invasive Treatments
4.4.2. Non-Invasive Treatments
4.4.3. Invasive Treatments
4.5. By Region (In Value %)
4.5.1. United States
4.5.2. Canada
4.5.3. Mexico
5. North America Urolithiasis Management Devices Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Boston Scientific
5.1.2. Cook Medical
5.1.3. Olympus Corporation
5.1.4. Medtronic
5.1.5. Dornier MedTech
5.1.6. Karl Storz
5.1.7. Richard Wolf GmbH
5.1.8. Siemens Healthineers
5.1.9. Stryker Corporation
5.1.10. Coloplast Group
5.1.11. EDAP TMS
5.1.12. Lumenis Ltd.
5.1.13. Bard Medical (BD)
5.1.14. STORZ MEDICAL AG
5.1.15. Quanta System
5.2. Cross Comparison Parameters
No. of Employees
Headquarters
Inception Year
Revenue
Product Portfolio Depth
Geographical Reach
R&D Focus
Partnerships and Collaborations
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. North America Urolithiasis Management Devices Market Regulatory Framework
6.1. Medical Device Classifications (FDA, Health Canada)
6.2. Compliance with ISO Standards
6.3. Certification Processes and Approvals
7. North America Urolithiasis Management Devices Future Market Size (In USD Mn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. North America Urolithiasis Management Devices Future Market Segmentation
8.1. By Device Type (In Value %)
8.2. By Technology (In Value %)
8.3. By End-User (In Value %)
8.4. By Treatment Type (In Value %)
8.5. By Region (In Value %)
9. North America Urolithiasis Management Devices Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
In this phase, we mapped out the entire ecosystem of stakeholders within the North American Urolithiasis Management Devices Market. Through extensive desk research, we identified crucial variables, such as device usage trends and patient demographics, to frame the market's key drivers and challenges.
Step 2: Market Analysis and Construction
We collected historical data related to the Urolithiasis Management Devices market. This analysis focused on hospital treatment rates, advancements in non-invasive procedures, and the revenue generated from these treatments. Our aim was to ensure accuracy in market size estimations by integrating data from credible government sources and market leaders.
Step 3: Hypothesis Validation and Expert Consultation
We developed hypotheses regarding the growth and challenges in the market, which were validated through consultations with industry experts. Interviews with device manufacturers and healthcare professionals provided operational insights that helped refine our market projections.
Step 4: Research Synthesis and Final Output
We engaged with key manufacturers to validate our data on device performance, product adoption, and treatment outcomes. This bottom-up approach allowed us to ensure that our final market analysis is comprehensive and accurate, offering a reliable view of the North American Urolithiasis Management Devices market.
Frequently Asked Questions
01. How big is the North America Urolithiasis Management Devices market?
The North America Urolithiasis Management Devices market was valued at USD 385 Mn, driven by increasing cases of kidney stones and advancements in non-invasive treatment technologies.
02. What are the challenges in the North America Urolithiasis Management Devices market?
Challenges in the North America Urolithiasis Management Devices market include the high costs of advanced urology devices and technical barriers associated with device handling. Additionally, the strict regulatory approval process for new devices poses a challenge for manufacturers.
03. Who are the major players in the North America Urolithiasis Management Devices market?
Major players in the North America Urolithiasis Management Devices market include Boston Scientific, Cook Medical, Olympus Corporation, Dornier MedTech, and Karl Storz. These companies dominate due to their innovative product portfolios and strong presence in key markets.
04. What are the growth drivers of the North America Urolithiasis Management Devices market?
Growth drivers in the North America Urolithiasis Management Devices market include the rising prevalence of kidney stones, technological advancements in lithotripsy and ureteroscopy, and increasing demand for outpatient procedures. Government healthcare initiatives are also supporting market expansion.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















