
U.S. Surgical Sutures Market Outlook to 2030
Region:North America
Author(s):Shubham
Product Code:KROD2555
November 2024
89
About the Report
U.S. Surgical Sutures Market Overview
- The U.S. Surgical Sutures market is valued at USD 1.95 billion, based on a five-year historical analysis. This market is primarily driven by advancements in minimally invasive surgical techniques, as well as the growing number of surgical procedures due to an aging population. With the increasing incidence of chronic diseases and rising demand for cosmetic surgeries, the surgical sutures market is experiencing steady growth. In addition, the focus on wound care management, particularly in trauma and post-surgical recovery, has elevated the importance of high-quality suturing techniques across healthcare facilities in the U.S.
- The market dominance is largely concentrated in major metropolitan areas such as New York, Los Angeles, and Chicago due to the high density of healthcare providers, hospitals, and advanced surgical centers. These regions boast a high concentration of healthcare professionals and well-established medical infrastructure, making them key contributors to market growth. Additionally, the governments continued investment in healthcare infrastructure and increasing healthcare expenditure support the market's expansion.
- The Food and Drug Administration (FDA) has established stringent regulations and safety standards for surgical sutures to ensure they meet performance criteria. In 2022, the FDA introduced new guidelines for biodegradable sutures, pushing manufacturers to adopt advanced materials that minimize tissue reaction and promote faster healing. Compliance with these regulations is a critical aspect for manufacturers seeking to maintain a competitive edge in the U.S. market.

U.S. Surgical Sutures Market Segmentation
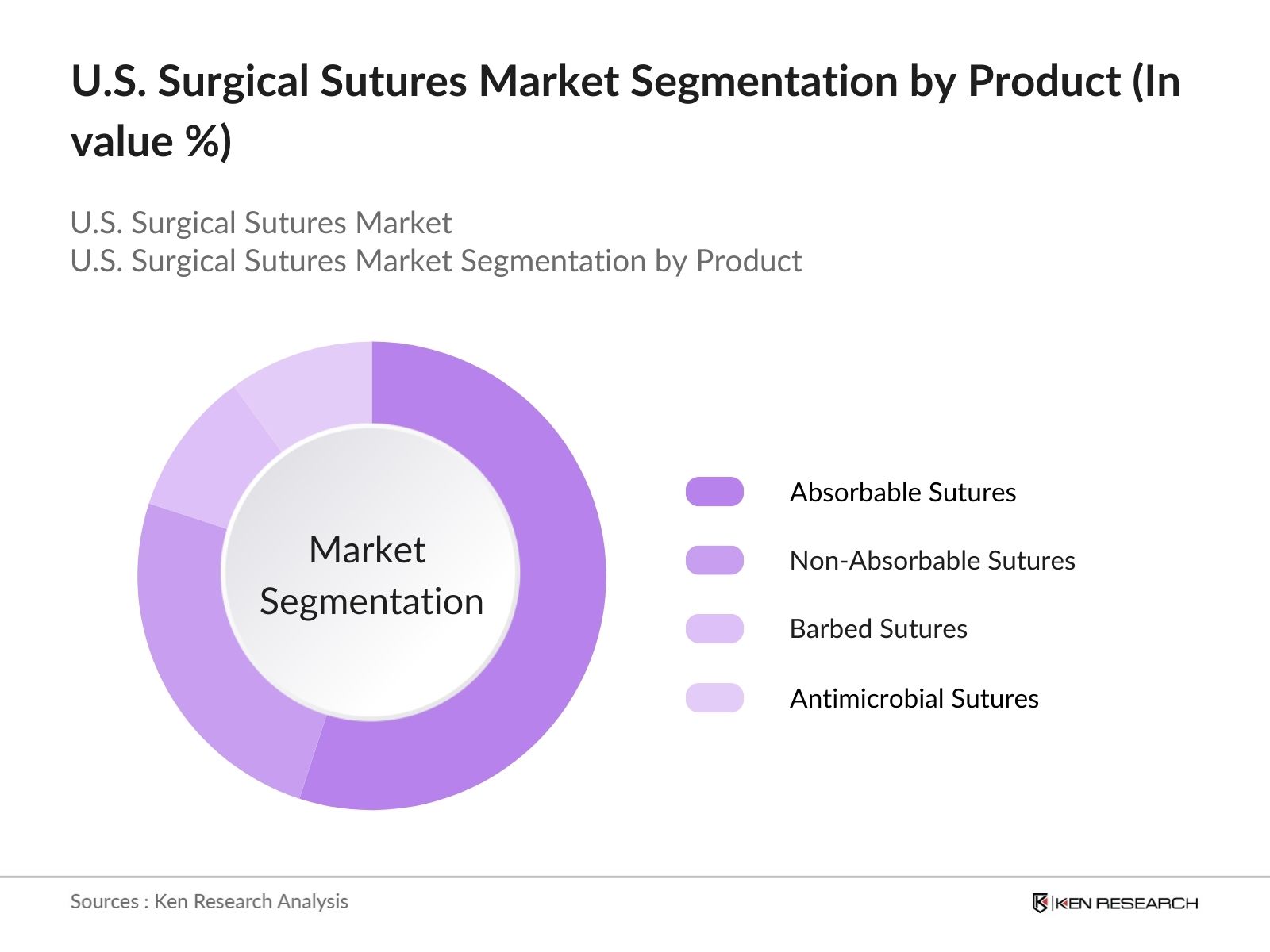
- By Product Type: The market is segmented by product type into absorbable sutures, non-absorbable sutures, barbed sutures, and antimicrobial sutures. Absorbable sutures have a dominant market share due to their use in internal tissue repairs, which dissolve over time and eliminate the need for suture removal. Antimicrobial sutures, which reduce the risk of infection at the suture site, are gaining traction, especially in high-risk surgeries. Major companies like Ethicon (Johnson & Johnson) and Medtronic lead in developing innovative sutures with advanced materials that promote faster healing.
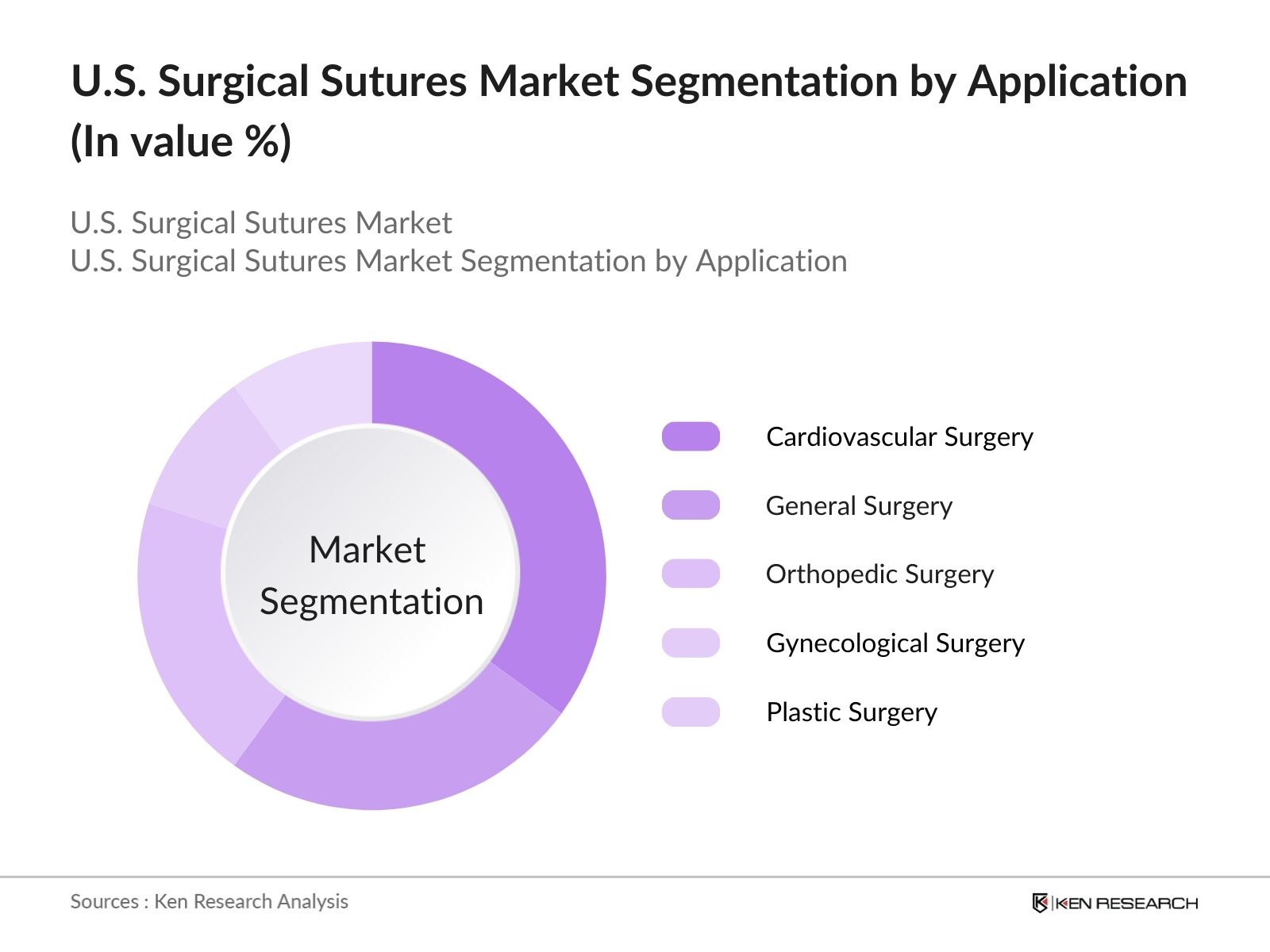
- By Application: The market is segmented by application into cardiovascular surgery, general surgery, gynecological surgery, orthopedic surgery, and plastic surgery. Cardiovascular surgery holds the largest market share due to the rising number of heart surgeries, including bypass and valve replacement procedures. The use of specialized sutures that provide excellent knot security and minimize tissue damage during critical surgeries drives their adoption in cardiac surgery. Plastic surgery is also growing significantly, with sutures designed for cosmetic precision playing a vital role in aesthetic outcomes.
U.S. Surgical Sutures Market Competitive Landscape
The market is dominated by key players who focus on continuous innovation, R&D, and strategic partnerships to strengthen their market positions. Companies like Ethicon and Medtronic lead the market with a strong emphasis on providing high-performance sutures for various surgical specialties. Other notable players include Smith & Nephew and B. Braun, which focus on enhancing wound closure technologies and expanding their product portfolios through mergers and acquisitions.
|
Company Name |
Establishment Year |
Headquarters |
Revenue (2023) |
Employees |
Key Product |
R&D Investment |
Key Clients |
Partnerships |
|
Ethicon (J&J) |
1887 |
New Jersey, USA |
||||||
|
Medtronic |
1949 |
Minnesota, USA |
||||||
|
Smith & Nephew |
1856 |
London, UK |
||||||
|
B. Braun |
1839 |
Melsungen, Germany |
||||||
|
Teleflex Medical |
1943 |
Pennsylvania, USA |
U.S. Surgical Sutures Industry Analysis
Growth Drivers
- Increasing Surgical Procedures (Cardiovascular, Orthopedic, Plastic): The number of surgical procedures in the U.S. has been steadily rising, driven by an aging population and the prevalence of chronic diseases. In 2022, approximately 1.4 million cardiovascular surgeries were performed, as reported by the CDC. Orthopedic procedures, including joint replacements, totaled over 1 million surgeries annually, according to the American Academy of Orthopedic Surgeons. The demand for plastic surgery also increased, with over 15.6 million cosmetic procedures performed in 2023. These procedures require different types of sutures, propelling the markets demand for both conventional and advanced suturing materials.
- Adoption of Minimally Invasive Surgeries: Minimally invasive surgeries (MIS) have gained momentum due to their reduced recovery times and lower complication rates. The National Institutes of Health reported that in 2023, over 30% of all surgical procedures in the U.S. were minimally invasive. Additionally, in 2023, 1.6 million cosmetic surgical procedures were conducted, alongside nearly 25.4 million minimally invasive procedures. MIS techniques, particularly in cardiovascular and orthopedic surgeries, often require specialized sutures that are less intrusive, leading to increased demand for absorbable and antimicrobial sutures. The expansion of MIS is expected to increase the need for advanced suturing techniques that accommodate these surgeries.
- Technological Advancements in Suturing Techniques: Technological innovations in suturing have contributed to the adoption of automated suturing devices and barbed sutures, enhancing procedural efficiency and reducing wound closure time. In 2023, the American Hospital Association reported a substantial increase in hospitals adopting robotic-assisted surgical systems, which often require precise suturing mechanisms. Additionally, sutures embedded with antimicrobial coatings, reducing infection rates by nearly 38%, have become more prevalent in high-risk surgeries such as cardiovascular and orthopedic procedures. These advancements are anticipated to drive significant growth in the U.S. sutures market.
Market Challenges
- Regulatory Hurdles (FDA Guidelines): The U.S. Food and Drug Administration (FDA) has stringent guidelines for the approval of new suture materials, especially those incorporating novel technologies such as antimicrobial coatings and bioresorbable materials. The FDA approval process for Class III medical devices, which includes most advanced sutures, can take several years, adding to the developmental costs for manufacturers. As of 2024, there are ongoing delays in approval pathways for over 100 new surgical devices, impacting the markets ability to bring innovative suture solutions to surgeons promptly.
- High Cost of Advanced Sutures (Absorbable, Antimicrobial): While advanced sutures such as absorbable and antimicrobial variants offer significant clinical benefits, their production costs remain high. Absorbable sutures, commonly used in surgeries to reduce infection rates, are often 34 times more expensive than traditional non-absorbable alternatives, according to Medicare data from 2023. The adoption of these sutures in low-budget healthcare settings, such as public hospitals and smaller surgical centers, remains a challenge due to these cost disparities, limiting their overall market penetration.
U.S. Surgical Sutures Market Future Outlook
Over the next five years, the U.S. Surgical Sutures market is expected to witness moderate growth, driven by continuous advancements in suture materials, increasing demand for minimally invasive surgeries, and the growing aging population. The integration of smart sutures with sensors for real-time wound monitoring is anticipated to revolutionize post-operative care, providing surgeons with enhanced insights into patient recovery. Furthermore, the rise in surgical tourism within the U.S., particularly for cosmetic procedures, will continue to bolster market growth.
Future Market Opportunities
- Expansion in Ambulatory Surgery Centers (ASCs): The growing number of ambulatory surgery centers (ASCs) presents a significant opportunity for the surgical sutures market. In 2022, there were over 6,000 ASCs in the U.S., and this number is expected to grow as more patients seek outpatient surgeries. ASCs provide a cost-effective alternative to traditional hospitals, and the demand for high-quality sutures is expected to increase as more procedures are performed in these facilities.
- Adoption of Biodegradable Sutures in Orthopedic Surgeries: The orthopedic surgery sector offers substantial growth opportunities for biodegradable sutures. As the U.S. population ages, the number of joint replacement surgeries and other orthopedic procedures is increasing. Biodegradable sutures, which minimize the need for suture removal and reduce the risk of long-term complications, are gaining popularity in this sector, providing a lucrative opportunity for manufacturers.
Scope of the Report
|
By Product Type |
Absorbable Sutures Non-Absorbable Sutures Barbed Sutures Antimicrobial Sutures |
|
By Application |
Cardiovascular Surgery General Surgery Orthopedic Surgery Gynecological Surgery Plastic Surgery |
|
By End User |
Hospitals Ambulatory Surgery Centers Specialized Surgical Clinics |
|
By Material |
Specialized Surgical Clinics Natural Sutures (Catgut, Silk) Synthetic Sutures (Nylon, PGA) |
|
By Region |
North-East Midwest West Coast Southern States |
Products
Key Target Audience
Hospitals and Healthcare Providers
Surgical Centers
Medical Device Manufacturers
Wound Care Specialists
Government and Regulatory Bodies (e.g., FDA, CDC)
Investment and Venture Capitalist Firms
Banks and Financial Institutes
Research and Development Institutes
Medical Device Distributors
Companies
Major Players in the Market
Ethicon (Johnson & Johnson)
Medtronic
Smith & Nephew
B. Braun
Teleflex Medical
Boston Scientific
Surgical Specialties Corporation
DemeTECH Corporation
Peters Surgical
Sutures India
Apollo Endosurgery
Zimmer Biomet
Integra LifeSciences
Dolphin Sutures
Riverpoint Medical
Table of Contents
1. U.S. Surgical Sutures Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (USD, CAGR)
1.4. Market Segmentation Overview
2. U.S. Surgical Sutures Market Size (in USD Billion)
2.1. Historical Market Size (In Value)
2.2. Year-On-Year Growth Analysis (Market Expansion Metrics)
2.3. Key Market Developments and Milestones (Product Innovations, New Surgical Methods)
3. U.S. Surgical Sutures Market Analysis
3.1. Growth Drivers
3.1.1. Increasing Surgical Procedures (Cardiovascular, Orthopedic, Plastic)
3.1.2. Adoption of Minimally Invasive Surgeries
3.1.3. Technological Advancements in Suturing Techniques
3.2. Market Challenges
3.2.1. Regulatory Hurdles (FDA Guidelines)
3.2.2. High Cost of Advanced Sutures (Absorbable, Antimicrobial)
3.2.3. Risk of Surgical Site Infections (SSI)
3.3. Opportunities
3.3.1. Adoption of Biodegradable Sutures
3.3.2. Growing Use in Ambulatory Surgery Centers (ASCs)
3.3.3. Expanding Applications in Plastic and Cosmetic Surgeries
3.4. Trends
3.4.1. Integration of Smart Sutures with Real-Time Monitoring
3.4.2. Increasing Demand for Customized Sutures (Tailored to Procedure Type)
3.4.3. Use of Barbed Sutures in Specialized Surgeries
3.5. Regulatory Environment
3.5.1. FDA Approval Pathways for New Suture Materials
3.5.2. Guidelines on Biocompatibility and Antimicrobial Coatings
3.5.3. Government Health Initiatives and Impact on Surgical Procedures
3.6. SWOT Analysis (U.S. Surgical Sutures Market)
3.7. Stakeholder Ecosystem (Surgeons, Manufacturers, Hospitals, Healthcare Institutions)
3.8. Porters Five Forces Analysis (Supplier Power, Buyer Power, Competition)
3.9. Competitive Ecosystem (Top Companies, Strategic Initiatives)
4. U.S. Surgical Sutures Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Absorbable Sutures
4.1.2. Non-Absorbable Sutures
4.1.3. Barbed Sutures
4.1.4. Antimicrobial Sutures
4.2. By Application (In Value %)
4.2.1. Cardiovascular Surgery
4.2.2. General Surgery
4.2.3. Orthopedic Surgery
4.2.4. Gynecological Surgery
4.2.5. Plastic Surgery
4.3. By End User (In Value %)
4.3.1. Hospitals
4.3.2. Ambulatory Surgery Centers (ASCs)
4.3.3. Specialized Surgical Clinics
4.4. By Material Type (In Value %)
4.4.1. Natural Sutures (Catgut, Silk)
4.4.2. Synthetic Sutures (Polyglycolic Acid, Nylon)
4.5. By Region (In Value %)
4.5.1. Northeast
4.5.2. Midwest
4.5.3. South
4.5.4. West
5. U.S. Surgical Sutures Market Competitive Landscape
5.1. Major Players and Competitive Analysis
5.1.1. Ethicon (Johnson & Johnson)
5.1.2. Medtronic
5.1.3. Smith & Nephew
5.1.4. B. Braun
5.1.5. Teleflex Medical
5.1.6. Boston Scientific
5.1.7. Surgical Specialties Corporation
5.1.8. DemeTECH Corporation
5.1.9. Peters Surgical
5.1.10. Sutures India
5.1.11. Apollo Endosurgery
5.1.12. Zimmer Biomet
5.1.13. Integra LifeSciences
5.1.14. Dolphin Sutures
5.1.15. Riverpoint Medical
5.2. Cross Comparison Parameters (No. of Employees, Headquarters, Inception Year, Revenue, Key Products, R&D Investment, Strategic Initiatives, Key Clients)
5.3. Market Share Analysis (By Product, By Application)
5.4. Strategic Initiatives (Partnerships, R&D Investment, New Product Launches)
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Private Equity Investments
6. U.S. Surgical Sutures Market Regulatory Framework
6.1. FDA Guidelines on Sutures (Performance Standards, Material Approval)
6.2. Compliance Requirements (Biocompatibility, Antimicrobial Standards)
6.3. Certification Processes (ISO, CE Mark for Sutures)
7. U.S. Surgical Sutures Future Market Size (in USD Billion)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth (Minimally Invasive Surgeries, Biodegradable Materials)
8. U.S. Surgical Sutures Future Market Segmentation
8.1. By Product Type (In Value %)
8.1.1. Absorbable Sutures
8.1.2. Non-Absorbable Sutures
8.2. By Application (In Value %)
8.2.1. Cardiovascular Surgery
8.2.2. General Surgery
8.3. By End User (In Value %)
8.3.1. Hospitals
8.3.2. Ambulatory Surgery Centers (ASCs)
8.4. By Material Type (In Value %)
8.4.1. Natural Sutures
8.4.2. Synthetic Sutures
8.5. By Region (In Value %)
9. U.S. Surgical Sutures Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis (Hospital Procurement Departments, ASC Buyers)
9.3. Marketing Initiatives (Digital Strategies, B2B Promotions)
9.4. White Space Opportunity Analysis (New Product Launches, Underserved Markets)
Contact US
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves constructing an ecosystem map encompassing all major stakeholders within the U.S. Surgical Sutures market. This step is underpinned by extensive desk research, utilizing a combination of secondary and proprietary databases to gather comprehensive industry-level information. The primary objective is to identify and define the critical variables that influence market dynamics.
Step 2: Market Analysis and Construction
In this phase, we compile and analyze historical data pertaining to the U.S. Surgical Sutures market. This includes assessing market penetration, the ratio of sutures to surgical procedures, and the resultant revenue generation. Furthermore, an evaluation of product quality statistics is conducted to ensure the reliability and accuracy of the revenue estimates.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are developed and subsequently validated through computer-assisted telephone interviews (CATIS) with industry experts representing a diverse array of companies. These consultations provide valuable operational and financial insights directly from industry practitioners, which are instrumental in refining and corroborating the market data.
Step 4: Research Synthesis and Final Output
The final phase involves direct engagement with multiple suture manufacturers to acquire detailed insights into product segments, sales performance, consumer preferences, and other pertinent factors. This interaction serves to verify and complement the statistics derived from the bottom-up approach, thereby ensuring a comprehensive, accurate, and validated analysis of the U.S. Surgical Sutures market.
Frequently Asked Questions
1. How big is the U.S. Surgical Sutures Market?
The U.S. Surgical Sutures market was valued at USD 1.95 billion in 2023, driven by the increasing number of surgeries and advancements in wound closure technologies.
2. What are the challenges in the U.S. Surgical Sutures Market?
Challenges include the high cost of advanced suture materials and the risk of surgical site infections, which can affect patient outcomes and increase healthcare costs.
3. Who are the major players in the U.S. Surgical Sutures Market?
Key players in the market include Ethicon (Johnson & Johnson), Medtronic, B. Braun, Smith & Nephew, and Teleflex Medical. These companies dominate due to their extensive product portfolios and strong R&D investments.
4. What are the growth drivers of the U.S. Surgical Sutures Market?
The market is propelled by factors such as the increasing number of surgeries, advancements in suture materials, and the growing popularity of minimally invasive procedures that require specialized suturing techniques.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















