
USA Angioplasty Balloons Market Outlook to 2030
Region:North America
Author(s):Meenakshi Bisht
Product Code:KROD5987
December 2024
82
About the Report
USA Angioplasty Balloons Market Overview
- The USA Angioplasty Balloons Market is valued at USD 779 million, driven by advancements in medical technology, increasing cardiovascular disease prevalence, and a rise in minimally invasive procedures. The demand for effective treatment options for arterial blockages and the availability of advanced balloon types, such as drug-eluting and cutting balloons, contribute significantly to the market's growth.
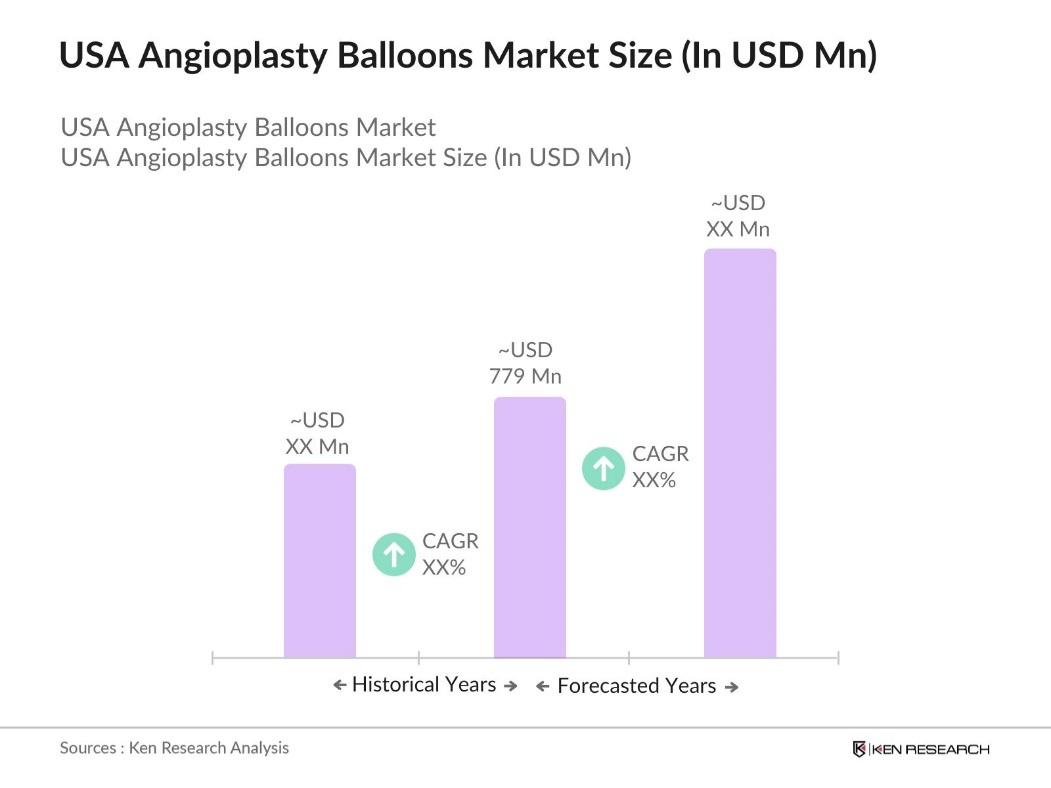
- In the USA, dominant regions include California, Texas, and Florida, where higher rates of cardiovascular diseases and a large elderly population increase the demand for angioplasty procedures. These states benefit from well-developed healthcare infrastructure and a high density of specialized cardiology centers, which supports the frequent use of angioplasty balloons.
- The reimbursement for a single major coronary artery angioplasty is approximately $631 as per the 2024 rates. This reimbursement rate is crucial for facilitating patient access to angioplasty, especially for older adults relying on Medicare coverage, although it does not fully cover the total cost.
USA Angioplasty Balloons Market Segmentation
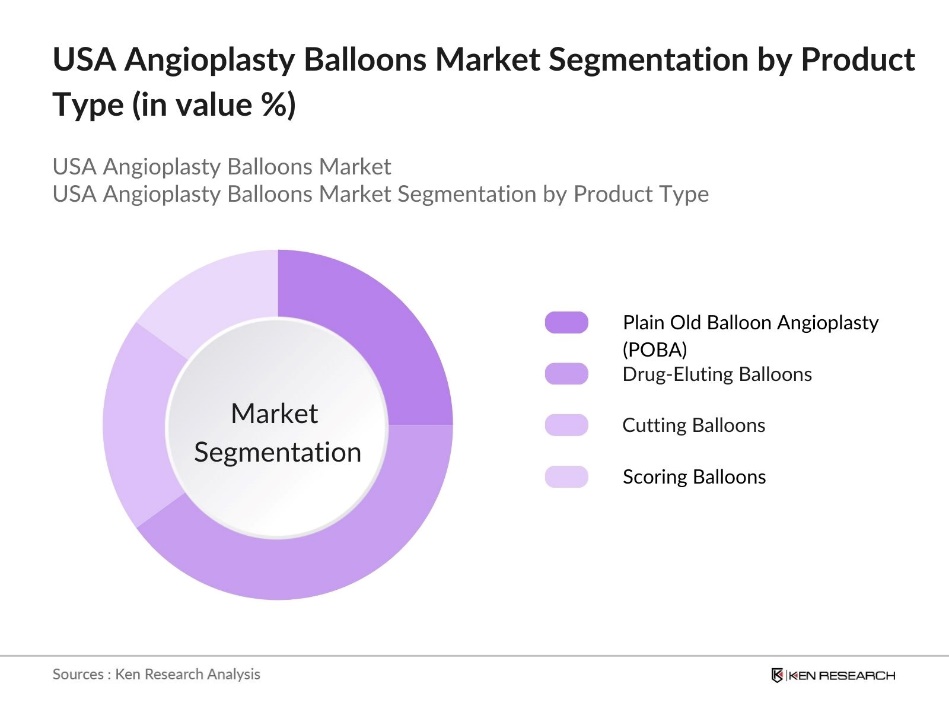
By Product Type: The market is segmented by product type into Plain Old Balloon Angioplasty (POBA), Drug-Eluting Balloons, Cutting Balloons, and Scoring Balloons. Drug-eluting balloons hold a significant share in this segment due to their effectiveness in reducing restenosis rates post-procedure. Their ability to deliver localized medication to the arterial site makes them a preferred option among healthcare providers, especially for patients with high recurrence risks.
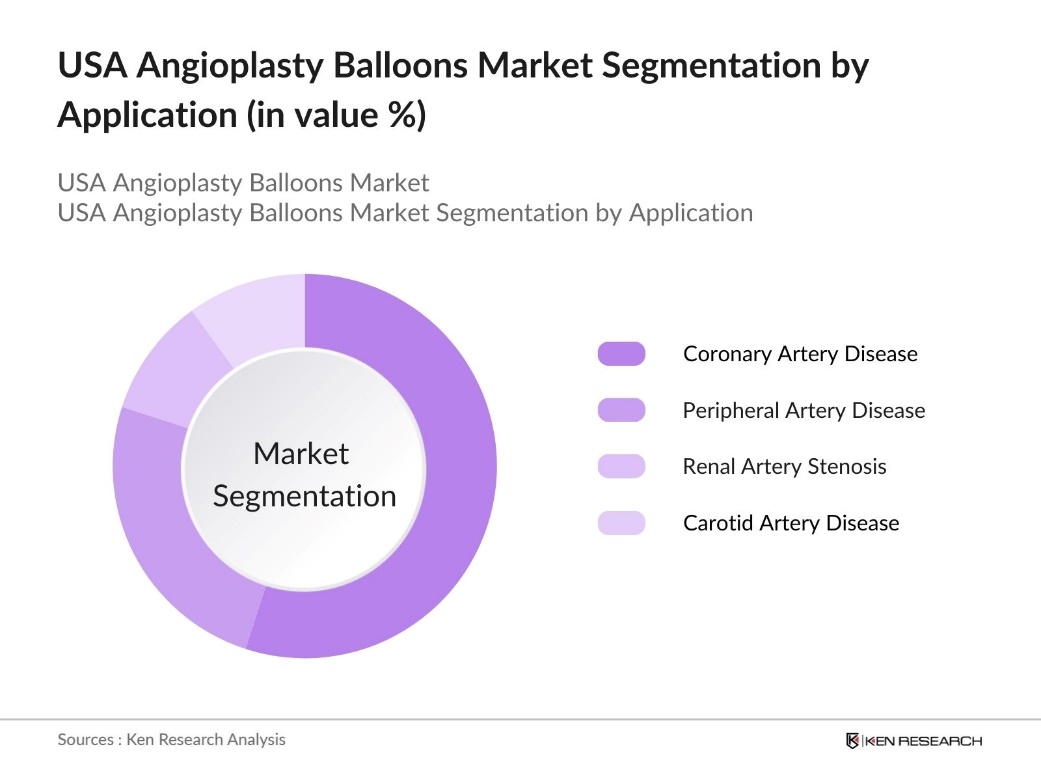
By Application: The market is segmented by application into Coronary Artery Disease, Peripheral Artery Disease, Renal Artery Stenosis, and Carotid Artery Disease. Coronary artery disease applications dominate due to the high incidence of this condition and the effectiveness of angioplasty in alleviating symptoms and reducing major cardiac events. The increased usage of advanced angioplasty balloons, especially in coronary interventions, drives demand in this application.
USA Angioplasty Balloons Market Competitive Landscape
The USA Angioplasty Balloons Market is consolidated with key players such as Boston Scientific Corporation, Medtronic plc, and Abbott Laboratories, dominating due to their extensive product portfolios and R&D investment in innovative balloon technologies. This market structure emphasizes the influence of established brands with broad geographic reach and strong distribution networks.
USA Angioplasty Balloons Industry Analysis
Growth Drivers
- Rising Prevalence of Cardiovascular Diseases: According to the CDC, the total number of deaths from heart disease in 2023 was approximately 680,909, making it the leading cause of death in the United States. This figure has led to an increased demand for advanced angioplasty solutions, including balloon catheters, as healthcare providers seek to manage high volumes of CVD-related cases. Factors like diet, lifestyle, and sedentary habits are fueling this trend, and angioplasty procedures remain critical in reducing mortality and morbidity linked to CVD.
- Technological Advancements in Balloon Catheters: The approval of a specific device, the AGENT Paclitaxel-Coated Balloon Catheter, which was approved in 2024, by Boston Scientific to treat coronary in-stent restenosis (ISR). These advancements have made newer balloon models more durable and effective for complex procedures, including drug-coated balloons that minimize restenosis. The FDA has consistently highlighted that the number of new approvals has increased due to refined materials and coatings used in balloon catheters, contributing to overall market growth.
- Increased Government and Private Health Spending (Healthcare Budget Allocation): The U.S. government and private health insurers have allocated substantial resources toward cardiovascular care, significantly supporting treatments like angioplasty balloons due to their role in minimally invasive, life-saving procedures. This rise in spending aligns with growing cardiovascular health demands, driving further advancements in angioplasty solutions and improving access to effective, cutting-edge treatments across healthcare facilities.
Market Challenges
- High Cost of Procedures (Average Procedure Cost): The high cost of angioplasty procedures in the U.S. poses significant accessibility challenges, particularly affecting uninsured or underinsured patients. This financial burden limits the adoption of angioplasty balloons in economically constrained healthcare facilities, creating barriers to providing equitable access to advanced cardiovascular care.
- Stringent FDA Regulations (Regulatory Compliance Costs): Bringing a new angioplasty balloon to market involves high regulatory compliance costs due to the FDA's stringent safety and efficacy requirements. This extensive approval process often delays product commercialization and places a heavy financial strain on smaller companies, limiting competition and innovation within the sector.
USA Angioplasty Balloons Market Future Outlook
Over the next five years, the USA Angioplasty Balloons Market is expected to experience steady growth, driven by increasing adoption of minimally invasive procedures, technological advancements in balloon designs, and rising healthcare spending. The market's expansion will be further propelled by an aging population that requires advanced cardiovascular care, along with the development of bioresorbable balloons, which offer safer alternatives to traditional devices. Increasing collaboration among key players to enhance product accessibility across various healthcare settings will support this growth.
Market Opportunities
- Expansion in Rural Healthcare Access (Rural Healthcare Infrastructure): Limited access to specialized healthcare in rural areas has spurred government efforts to improve rural healthcare infrastructure, with a focus on expanding cardiovascular care. These initiatives are set to increase demand for angioplasty balloons, as hospitals in rural regions receive the necessary equipment and resources to provide advanced cardiovascular treatments.
- Emerging Minimally Invasive Techniques (Patents Filed): Recent advancements in minimally invasive cardiovascular techniques present significant opportunities for the angioplasty balloon market. These innovations enhance procedural efficiency and reduce patient recovery times, making angioplasty balloons more widely adopted in clinical settings focused on improving patient outcomes with minimally invasive options.
Scope of the Report
|
Product Type |
Plain Old Balloon Angioplasty (POBA) Drug-Eluting Balloons Cutting Balloons Scoring Balloons |
|
Material |
Nylon Polyurethane Silicone Elastomers |
|
Application |
Coronary Artery Disease Peripheral Artery Disease Renal Artery Stenosis Carotid Artery Disease |
|
End-User |
Hospitals Ambulatory Surgical Centers Specialty Clinics |
|
Region |
Northeast Midwest South West |
Products
Key Target Audience
Hospitals and Surgical Centers
Medical Device Import/Export Firms
Specialty Medical Device Manufactures Industry
Medical Device Manufacturers
Government and Regulatory Bodies (FDA, CMS)
Investors and venture capital Firms
Banks and Financial Institutions
Companies
Players Mentioned in the Report
Boston Scientific Corporation
Medtronic plc
Abbott Laboratories
Terumo Corporation
Cook Medical
Koninklijke Philips N.V.
B. Braun Melsungen AG
AngioDynamics, Inc.
BIOTRONIK SE & Co. KG
Spectranetics Corporation
Table of Contents
1. USA Angioplasty Balloons Market Overview
1.1 Definition and Scope
1.2 Market Taxonomy
1.3 Market Growth Rate and Dynamics
1.4 Market Segmentation Overview
2. USA Angioplasty Balloons Market Size (In USD Mn)
2.1 Historical Market Size
2.2 Year-On-Year Growth Analysis
2.3 Key Market Developments and Milestones
3. USA Angioplasty Balloons Market Analysis
3.1 Growth Drivers
3.1.1 Rising Prevalence of Cardiovascular Diseases (CVD Cases per 100,000)
3.1.2 Technological Advancements in Balloon Catheters (New Product Approvals)
3.1.3 Increased Government and Private Health Spending (Healthcare Budget Allocation)
3.1.4 Growing Geriatric Population (Population Aged 65+)
3.2 Market Challenges
3.2.1 High Cost of Procedures (Average Procedure Cost)
3.2.2 Stringent FDA Regulations (Regulatory Compliance Costs)
3.2.3 Risk of Procedure-Related Complications (Incidence Rates)
3.2.4 Limited Skilled Workforce (No. of Certified Interventional Cardiologists)
3.3 Opportunities
3.3.1 Expansion in Rural Healthcare Access (Rural Healthcare Infrastructure)
3.3.2 Emerging Minimally Invasive Techniques (Patents Filed)
3.3.3 Strategic Partnerships for Product Development (Number of Alliances)
3.3.4 Increasing Focus on Ambulatory Surgery Centers (ASC Growth Rate)
3.4 Trends
3.4.1 Adoption of Drug-Eluting Balloons (Market Penetration Rate)
3.4.2 Advancements in Biodegradable Balloons (Innovation Index)
3.4.3 Integration with Imaging Technologies (Tech Adoption Rates)
3.4.4 Increased Use of Cost-Efficient PTA Balloons (Utilization Rates)
3.5 Government Regulations
3.5.1 FDA Approval Process (Clinical Trial Requirements)
3.5.2 Medicare and Medicaid Coverage (Reimbursement Rates)
3.5.3 Medical Device Excise Tax (Tax Rate and Impact)
3.5.4 Hospital Outpatient Department (HOPD) Reimbursement Policies
3.6 SWOT Analysis
3.7 Value Chain Analysis
3.8 Porters Five Forces Analysis
3.9 Competition Ecosystem
4. USA Angioplasty Balloons Market Segmentation
4.1 By Product Type (In Value %)
4.1.1 Plain Old Balloon Angioplasty (POBA)
4.1.2 Drug-Eluting Balloons
4.1.3 Cutting Balloons
4.1.4 Scoring Balloons
4.2 By Material (In Value %)
4.2.1 Nylon
4.2.2 Polyurethane
4.2.3 Silicone Elastomers
4.3 By Application (In Value %)
4.3.1 Coronary Artery Disease
4.3.2 Peripheral Artery Disease
4.3.3 Renal Artery Stenosis
4.3.4 Carotid Artery Disease
4.4 By End-User (In Value %)
4.4.1 Hospitals
4.4.2 Ambulatory Surgical Centers
4.4.3 Specialty Clinics
4.5 By Region (In Value %)
4.5.1 Northeast
4.5.2 Midwest
4.5.3 South
4.5.4 West
5. USA Angioplasty Balloons Market Competitive Analysis
5.1 Detailed Profiles of Major Companies
5.1.1 Boston Scientific Corporation
5.1.2 Medtronic plc
5.1.3 Abbott Laboratories
5.1.4 Terumo Corporation
5.1.5 Cardinal Health, Inc.
5.1.6 Cook Medical
5.1.7 Koninklijke Philips N.V.
5.1.8 B. Braun Melsungen AG
5.1.9 C. R. Bard, Inc.
5.1.10 AngioDynamics, Inc.
5.1.11 BIOTRONIK SE & Co. KG
5.1.12 Spectranetics Corporation
5.1.13 Teleflex Incorporated
5.1.14 OrbusNeich Medical
5.1.15 Merit Medical Systems
5.2 Cross Comparison Parameters (Revenue, R&D Investment, Product Portfolio, FDA Clearances, Market Share, No. of Employees, Geographical Reach, Headquarters Location)
5.3 Market Share Analysis
5.4 Strategic Initiatives
5.5 Mergers and Acquisitions
5.6 Investment Analysis
5.7 Venture Capital Funding
5.8 Government Grants
5.9 Private Equity Investments
6. USA Angioplasty Balloons Market Regulatory Framework
6.1 FDA Approval and Clearance Requirements
6.2 Quality and Safety Standards
6.3 Clinical Trial Compliance Guidelines
6.4 Certification and Licensing Processes
7. USA Angioplasty Balloons Future Market Size (In USD Mn)
7.1 Future Market Size Projections
7.2 Key Factors Driving Future Market Growth
8. USA Angioplasty Balloons Future Market Segmentation
8.1 By Product Type (In Value %)
8.2 By Material (In Value %)
8.3 By Application (In Value %)
8.4 By End-User (In Value %)
8.5 By Region (In Value %)
9. USA Angioplasty Balloons Market Analysts Recommendations
9.1 TAM/SAM/SOM Analysis
9.2 Customer Cohort Analysis
9.3 Strategic Marketing Initiatives
9.4 White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The initial step in this research involves mapping all major stakeholders within the USA Angioplasty Balloons Market. This is achieved through extensive desk research, utilizing secondary and proprietary databases to gather comprehensive market insights and identify critical variables that influence the market.
Step 2: Market Analysis and Construction
This phase compiles historical data on market penetration and product demand. The analysis focuses on evaluating market share distribution among key product types and applications, as well as revenue generation patterns in different geographic areas.
Step 3: Hypothesis Validation and Expert Consultation
Hypotheses regarding market trends and growth factors are validated through consultations with industry experts. These insights are gathered via computer-assisted telephone interviews (CATIs) with professionals from top companies, providing financial and operational data.
Step 4: Research Synthesis and Final Output
The final stage involves synthesizing insights from industry interactions and bottom-up data approaches to verify and refine our projections, ensuring an accurate and validated analysis of the USA Angioplasty Balloons Market.
Frequently Asked Questions
01. How big is the USA Angioplasty Balloons Market?
The USA Angioplasty Balloons Market is valued at USD 779 million, with demand driven by the high incidence of cardiovascular diseases and advanced treatment options.
02. What are the challenges in the USA Angioplasty Balloons Market?
Challenges in USA Angioplasty Balloons Market include high procedure costs, stringent regulatory requirements, and the need for skilled cardiologists, which can limit the accessibility of advanced angioplasty treatments.
03. Who are the major players in the USA Angioplasty Balloons Market?
Key players in USA Angioplasty Balloons Market include Boston Scientific Corporation, Medtronic plc, Abbott Laboratories, Terumo Corporation, and Cook Medical, who dominate due to their extensive portfolios and innovation in angioplasty technology.
04. What are the growth drivers of the USA Angioplasty Balloons Market?
Growth drivers in USA Angioplasty Balloons Market include rising rates of cardiovascular diseases, advancements in balloon technology, and increasing healthcare expenditure, especially in cardiovascular care.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















