
USA Biopharma Contract Research Market Outlook to 2030
Region:North America
Author(s):Shivani Mehra
Product Code:KROD2135
December 2024
97
About the Report
USA Biopharma Contract Research Market Overview
- The USA Biopharma Contract Research Market is valued at USD 11.1 billion, driven by the surge in outsourcing by biopharma companies seeking cost-effective and efficient R&D solutions. The complexity of drug development and regulatory demands, coupled with high operational costs, are pushing biopharma companies to partner with specialized contract research organizations (CROs) for clinical trials, laboratory services, and data management. This market is expected to grow as biopharma companies increasingly rely on CROs for faster and more flexible R&D solutions.
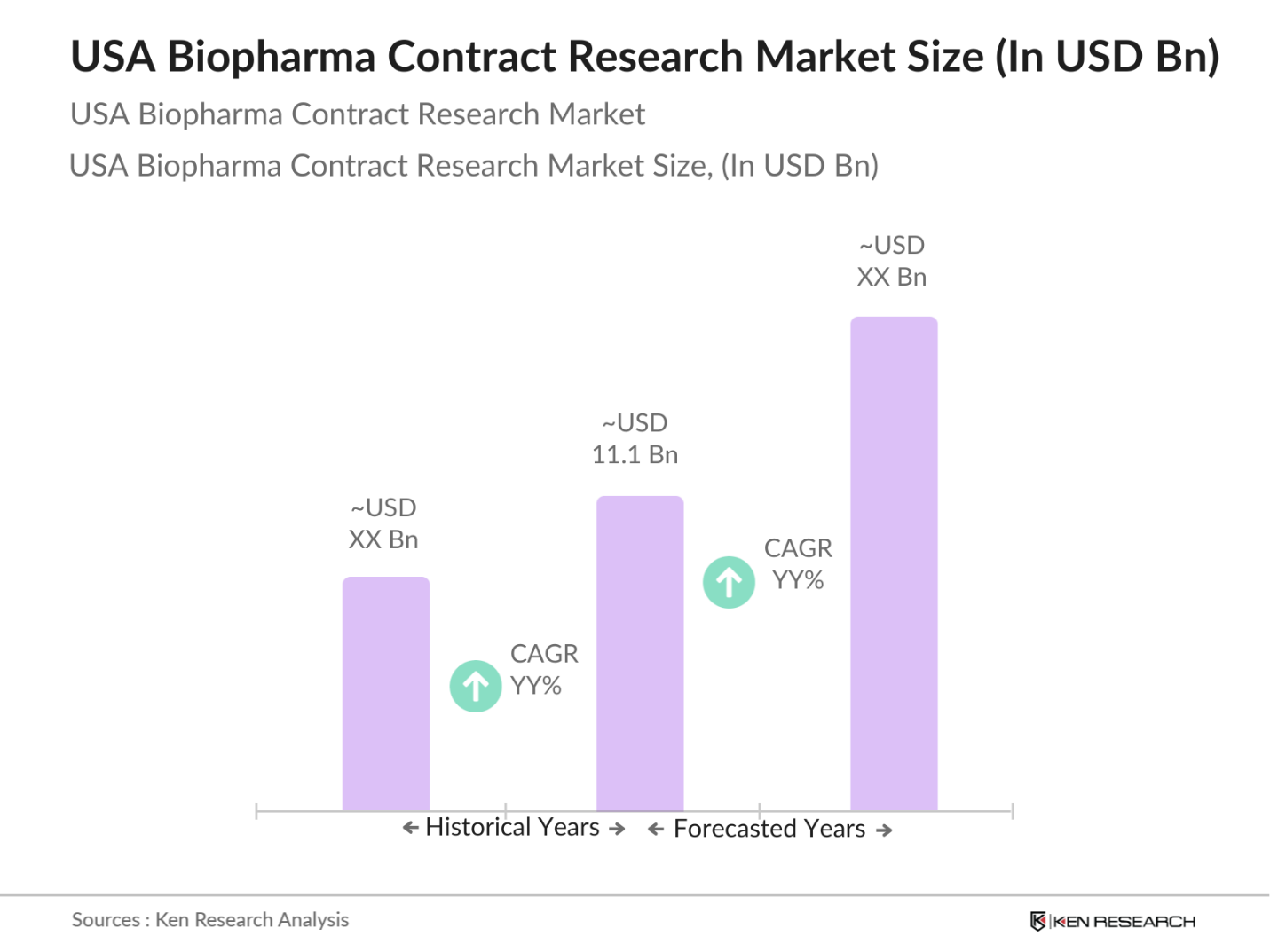
- The Northeast and West regions lead the USA Biopharma Contract Research Market, attributed to the high concentration of pharmaceutical companies, research institutions, and regulatory agencies. The Northeast, in particular, benefits from proximity to leading biopharma companies and access to a skilled workforce, while the West is experiencing growth in biotech hubs such as California.
- The FDAs strict drug approval and safety regulations continue to shape the biopharma contract research market. In 2023, the FDA approved 60 new drugs, many of which underwent extensive clinical trials conducted by contract research organizations. These regulatory requirements ensure that drugs meet high safety and efficacy standards, presenting both a challenge and an opportunity for CROs to support biopharma companies in navigating the approval process. CROs play a crucial role in ensuring compliance with FDA guidelines during drug development, clinical trials, and post-market surveillance.
USA Biopharma Contract Research Market Segmentation
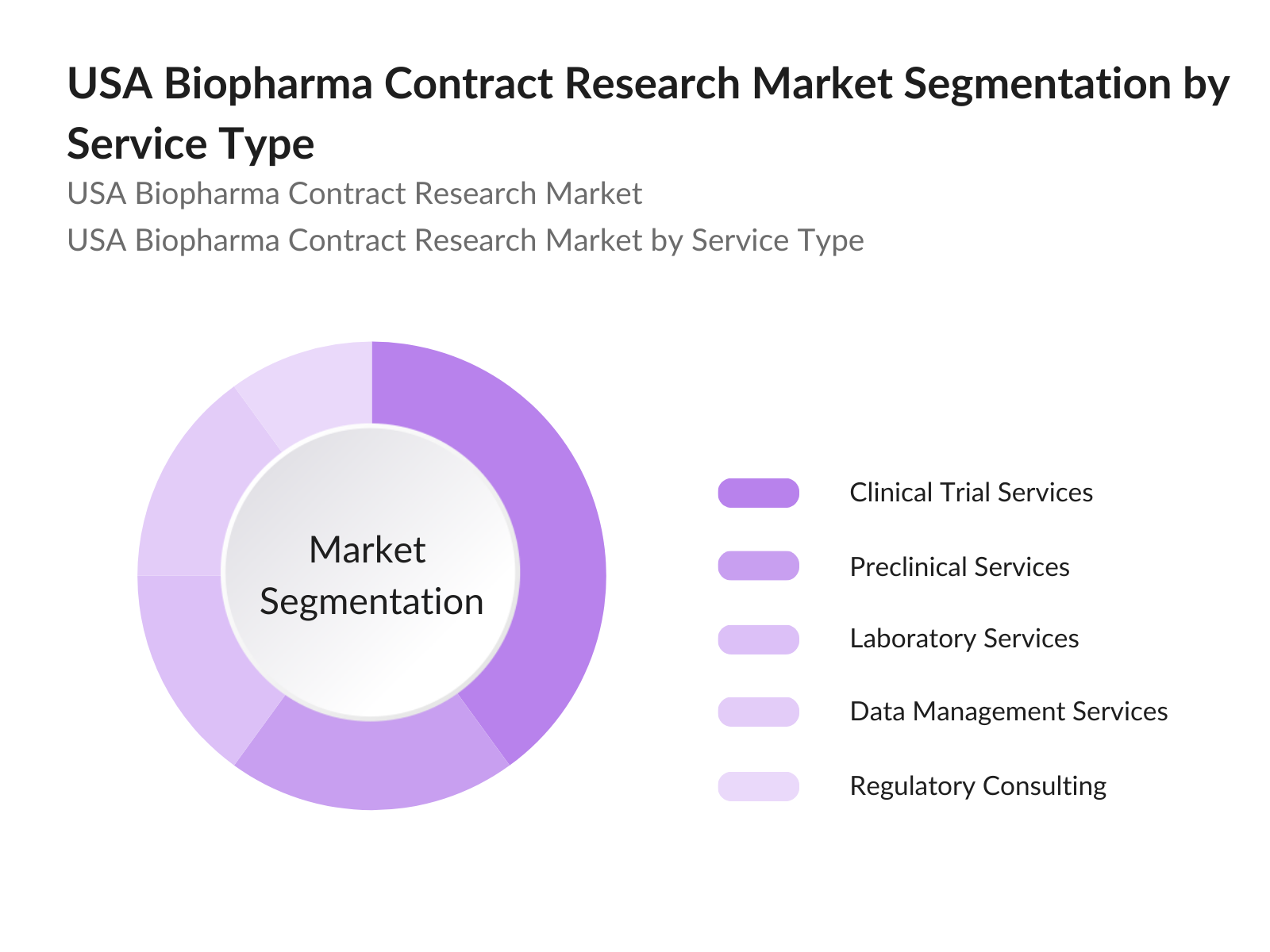
- By Service Type: The USA Biopharma Contract Research Market is segmented by service type into clinical trial services, preclinical services, laboratory services, data management services, and regulatory consulting. Clinical trial services dominate the market due to the increasing number of drugs in the development pipeline, as well as the high cost and complexity of conducting clinical trials in-house. CROs provide expertise and infrastructure that allow biopharma companies to focus on core R&D activities.
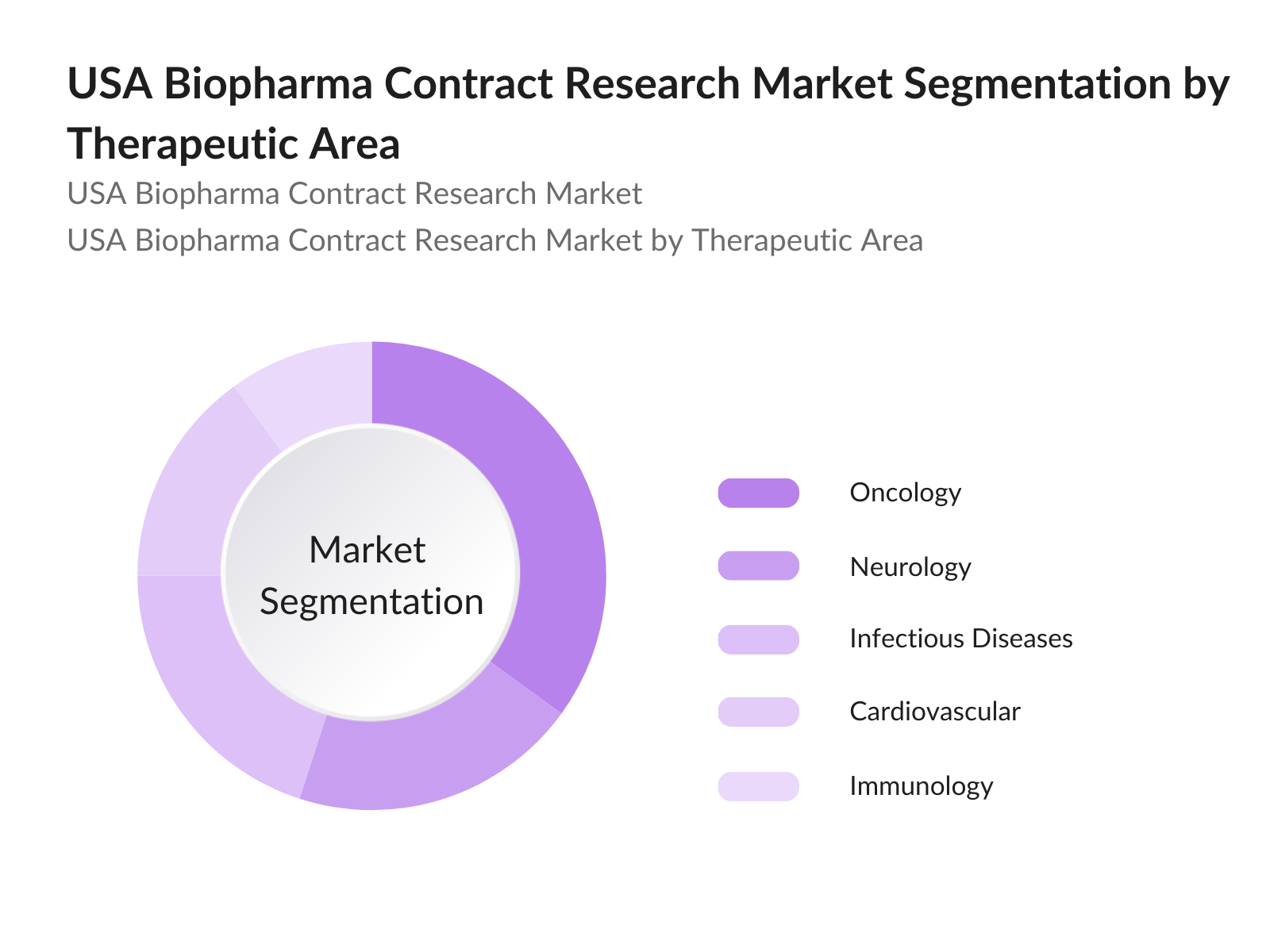
- By Therapeutic Area: The market segmentation by therapeutic area includes oncology, neurology, cardiovascular, infectious diseases, and immunology. Oncology dominates due to the high number of cancer-related drug development projects and the complexity of conducting oncology trials, which require specialized CRO services. The increasing incidence of cancer and the focus on personalized medicine are also major drivers for CRO services in oncology.
USA Biopharma Contract Research Market Competitive Landscape
The USA Biopharma Contract Research Market is highly competitive, with several global and regional players investing in advanced technologies and partnerships. Leading CROs like IQVIA and LabCorp Drug Development benefit from their extensive service portfolios, technological capabilities, and strong relationships with biopharma clients.
|
Company |
Establishment Year |
Headquarters |
Regional Presence |
Revenue ($ Bn) |
Employee Strength |
R&D Investment |
Specialized Services |
Data Security Certifications |
Strategic Partnerships |
|
IQVIA |
1982 |
North Carolina, USA |
- | - | - | - | - | - | - |
|
LabCorp Drug Development |
1969 |
North Carolina, USA |
- | - | - | - | - | - | - |
|
PPD, Inc. |
1985 |
North Carolina, USA |
- | - | - | - | - | - | - |
|
Charles River Laboratories |
1947 |
Massachusetts, USA |
- | - | - | - | - | - | - |
|
ICON Plc |
1990 |
Dublin, Ireland |
- | - | - | - | - | - | - |
USA Biopharma Contract Research Market Analysis
Market Growth Drivers
- Increased Outsourcing by Biopharma Companies: Outsourcing of research and development (R&D) activities by biopharma companies is increasing as they seek to reduce operational costs and speed up development timelines. In 2023 of R&D spending by major U.S. pharmaceutical companies is directed toward contract research organizations (CROs). This trend is largely due to the high costs associated with in-house R&D, with biopharma companies opting for CROs to manage preclinical and clinical research stages. These outsourced services help companies reduce overheads and access specialized expertise, especially in highly regulated areas like drug testing and clinical trials.
- Rise in Drug Development Activities: Drug development activities in the U.S. are increasing as pharmaceutical companies invest more in bringing new drugs to market. According to the U.S. Food and Drug Administration (FDA), over 50 new molecular entities (NMEs) were approved in 2023, reflecting the growing drug development pipeline. Biopharma companies are increasingly turning to CROs to handle the complexities of clinical trials, regulatory submissions, and drug testing. In 2023 of new drug candidates go through contract research organizations for clinical trials, underscoring the significant role CROs play in drug development and regulatory processes.
- Advancements in Biopharmaceutical R&D: Advancements in biopharmaceutical research and development (R&D) are driving growth in the contract research market. In 2023, U.S. biopharma companies are heavily investing in the development of gene therapies and personalized medicine, areas that require specialized research and clinical testing. The FDA approved 9 gene therapies in 2023, the highest in any given year, showing the growing emphasis on innovative treatments. CROs are essential for supporting these cutting-edge therapies, as they provide necessary infrastructure for conducting clinical trials, managing data, and ensuring regulatory compliance during the research process.
Market Challenges:
- High Operational Costs: Operational costs in the biopharma contract research market remain high, particularly for clinical trials. In 2023, the average cost for a clinical trial in the U.S. is estimated at $2 million per study. These high costs stem from the need for advanced technologies, extensive regulatory compliance, and extensive recruitment efforts for patient participation. Biopharma companies often face budget overruns during the course of these trials, which can limit the scope of outsourcing or the scale of research projects. These costs are a significant challenge, especially for smaller biopharma firms with limited budgets.
- Regulatory Compliance Complexities: Regulatory compliance remains a complex challenge for contract research organizations. With the FDAs evolving regulations for clinical trials, drug safety, and post-market surveillance, CROs must keep up with changes to ensure that biopharma clients remain compliant. In 2023, the FDA has introduced new rules for the reporting of adverse drug reactions, which increase the complexity of clinical trial management. The regulatory environment, with its focus on patient safety and ethical considerations, requires CROs to invest in regulatory expertise and robust systems to manage compliance, adding to the operational burden.
USA Biopharma Contract Research Market Future Outlook
The USA Biopharma Contract Research Market is projected to grow significantly over the next five years, driven by a focus on biologics and biosimilars, an increasing demand for decentralized trials, and the integration of AI in clinical research. Key growth areas will include oncology, infectious diseases, and personalized medicine, where advanced CRO services will play a vital role in accelerating drug development and ensuring regulatory compliance.
Market Opportunities:
- Growth in Biologics and Biosimilars Research: The U.S. market for biologics and biosimilars is expanding rapidly, creating opportunities for contract research organizations. In 2023, the FDA is expected to approve 12 new biosimilars, reflecting the increasing focus on biologic therapies as a treatment for complex diseases such as cancer and autoimmune disorders. Biopharma companies are increasingly turning to CROs to support the development of these biologics, particularly in the regulatory and clinical trial stages. As the demand for biologic drugs increases, CROs have a significant opportunity to provide specialized services, including clinical trials, regulatory support, and data management.
- Expansion of Specialized CRO Services: Specialized CRO services are expanding to meet the growing complexity of drug development. As biopharma companies focus more on niche therapies, including gene and cell therapies, there is an increasing demand for CROs with expertise in these areas. In 2023 of contract research organizations in the U.S. are focusing on specialized services, such as early-phase clinical trials for novel therapies and regulatory compliance for rare diseases. This market segment is expected to grow as biopharma companies seek expert partners to navigate the complexities of specialized research and development.
Scope of the Report
|
By Service Type |
Clinical Trial Services Preclinical Services Laboratory Services Data Management Services Regulatory Consulting |
|
By Therapeutic Area |
Oncology Infectious Diseases |
|
By Phase |
Phase I Phase II Phase III Phase IV |
|
By Sponsor Type |
Small and Medium Biopharma |
|
By Region |
North-East Midwest West Coast Southern States |
Products
Key Target Audience
Biopharma Companies
Government Research Institutions
Pharmaceutical Companies
Biotech Startups
Investments and Venture Capitalist Firms
Government and Regulatory Bodies(e.g., U.S. Food and Drug Administration)
University Research Departments
Healthcare Providers and Hospitals
Companies
Players mentioned in the report
IQVIA
LabCorp Drug Development
PPD, Inc.
PRA Health Sciences
Charles River Laboratories
ICON Plc
Medpace Holdings
Syneos Health
Parexel International Corporation
WuXi AppTec
Covance Inc.
Pharmaceutical Product Development, LLC
KCR
Worldwide Clinical Trials
Envigo
Table of Contents
1. USA Biopharma Contract Research Market Overview
1.1. Market Definition and Scope
1.2. Market Dynamics and Growth Drivers
1.3. Key Trends and Regional Insights
1.4. Regulatory Influence on Market Development
2. USA Biopharma Contract Research Market Size (USD Bn)
2.1. Current Market Valuation
2.2. Historical Market Size
2.3. Year-on-Year Growth Analysis
2.4. Key Developments and Milestones
3. USA Biopharma Contract Research Market Segmentation
3.1. By Service Type
3.1.1. Clinical Trial Services
3.1.2. Preclinical Services
3.1.3. Laboratory Services
3.1.4. Data Management Services
3.1.5. Regulatory Consulting
3.2. By Therapeutic Area
3.2.1. Oncology
3.2.2. Neurology
3.2.3. Cardiovascular
3.2.4. Infectious Diseases
3.2.5. Immunology
3.3. By Phase
3.3.1. Phase I
3.3.2. Phase II
3.3.3. Phase III
3.3.4. Phase IV
3.4. By Sponsor Type
3.4.1. Small and Medium Biopharma
3.4.2. Large Biopharma
3.4.3. Government/Academic
3.5. By Region
3.5.1. Northeast
3.5.2. Midwest
3.5.3. West Coast
3.5.4. Southern States
4. USA Biopharma Contract Research Market Competitive Landscape
4.1. Profiles of Key Players
4.1.1. IQVIA
4.1.2. LabCorp Drug Development
4.1.3. PPD, Inc.
4.1.4. Charles River Laboratories
4.1.5. ICON Plc
4.2. Market Share Analysis
4.3. Competitive Strategies
4.4. Strategic Partnerships and Collaborations
5. USA Biopharma Contract Research Market Analysis
5.1. Market Growth Drivers
5.1.1. Increased Outsourcing by Biopharma Companies
5.1.2. Rise in Drug Development Activities
5.1.3. Advancements in Biopharmaceutical R&D
5.2. Market Challenges
5.2.1. High Operational Costs
5.2.2. Regulatory Compliance Complexities
5.3. Market Opportunities
5.3.1. Growth in Biologics and Biosimilars Research
5.3.2. Expansion of Specialized CRO Services
6. USA Biopharma Contract Research Market Future Outlook
6.1. Projections and Future Market Size
6.2. Key Growth Areas for 2028
6.3. Technological Integration and Decentralized Trials
7. USA Biopharma Contract ResearchMarket Analysts Recommendations
7.1. Market Size and Valuation
7.2. Key Market Challenges
7.3. Major Players in the Market
7.4. Growth Drivers and Opportunities
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
This initial phase maps the key players, market drivers, and regulatory factors impacting the USA Biopharma Contract Research Market, leveraging data from proprietary databases.
Step 2: Market Analysis and Construction
Historical data on clinical trial volume, segment growth, and trends in drug development are analyzed to provide a comprehensive view of the market size and dynamics.
Step 3: Hypothesis Validation and Expert Consultation
Industry experts are consulted to validate assumptions regarding demand drivers, technological innovations, and regulatory requirements for CRO services.
Step 4: Research Synthesis and Final Output
The final stage synthesizes data and insights into a comprehensive report, ensuring a robust and validated analysis of the USA Biopharma Contract Research Market.
Frequently Asked Questions
01. How big is the USA Biopharma Contract Research Market?
The USA Biopharma Contract Research Market is valued at USD 11.1 billion, driven by biopharma companies increasing reliance on CROs for efficient and cost-effective R&D.
02. What are the challenges in the USA Biopharma Contract Research Market?
Challenges include high operational costs, regulatory compliance complexities, and data privacy concerns related to clinical trials and drug development.
03. Who are the major players in the USA Biopharma Contract Research Market?
Key players include IQVIA, LabCorp Drug Development, PPD, Charles River Laboratories, and ICON Plc, known for their specialized services and extensive biopharma partnerships.
04. What are the growth drivers of the USA Biopharma Contract Research Market?
Growth is driven by increased outsourcing, advancements in biologics research, and the need for specialized services in complex therapeutic areas like oncology and infectious diseases.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















