
USA Clinical Trials Management Systems Market Outlook to 2030
Region:North America
Author(s):Sanjna
Product Code:KROD9866
November 2024
99
About the Report
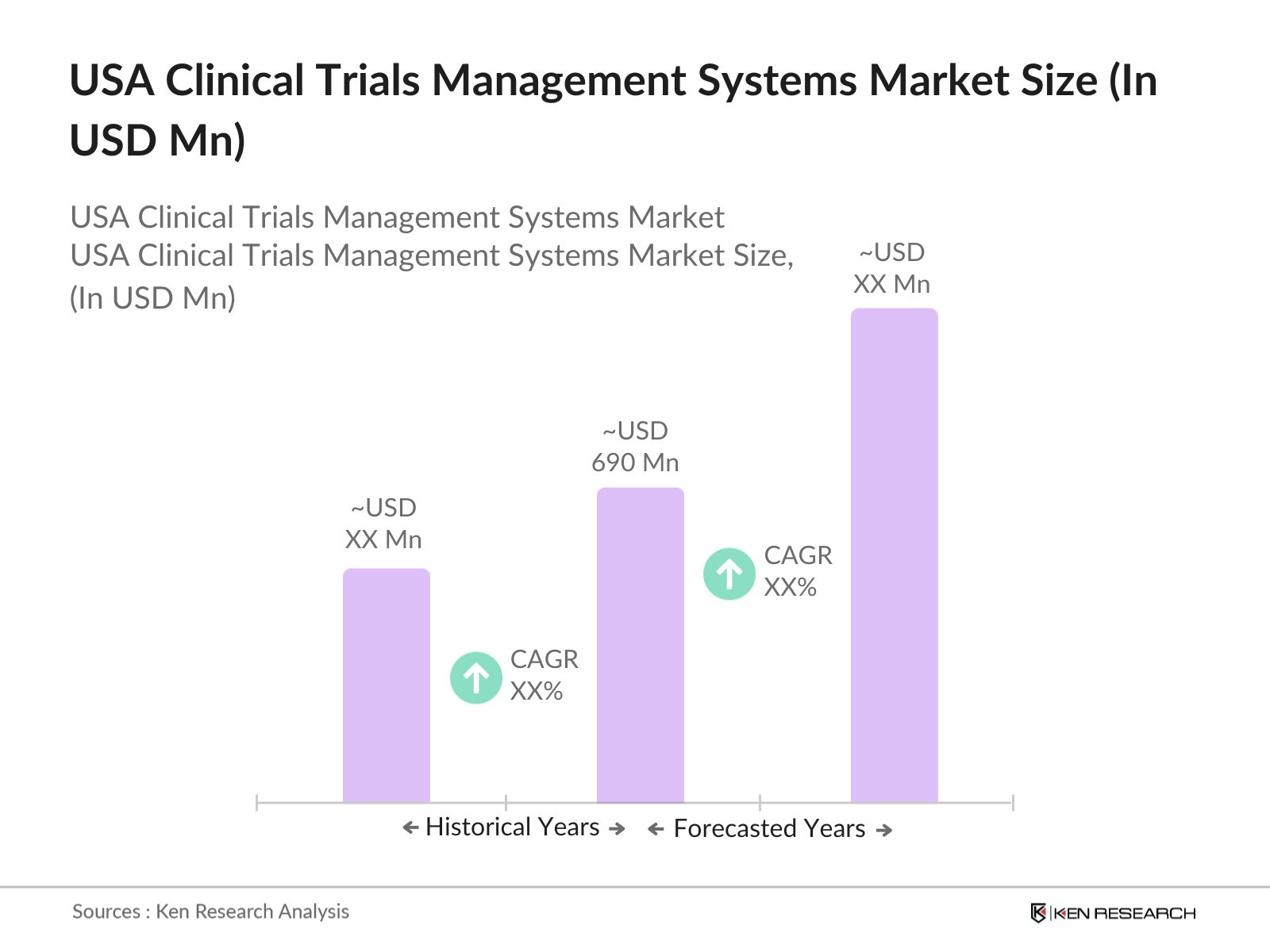
USA Clinical Trials Management Systems Market Overview
- The USA Clinical Trials Management Systems market is valued at USD 690 million, driven by the increasing complexity of clinical research, the need for efficient data management systems, and growing investment in drug development. The rise in personalized medicine and a focus on precision therapies have further fueled demand for advanced clinical trials management solutions. Companies are adopting innovative technologies, such as AI and machine learning, to streamline clinical trial processes and reduce timelines, which is a critical factor for the markets growth.
- The dominance of key cities, such as Boston, San Francisco, and New York, is attributed to the strong presence of biotechnology and pharmaceutical industries, along with access to top-tier research institutions. These cities serve as hubs for clinical trials, benefiting from an experienced workforce and proximity to regulatory bodies. The geographical concentration of funding and resources makes these cities pivotal in shaping the clinical trials landscape in the USA.
- The U.S. Food and Drug Administration (FDA) has been actively updating clinical trial guidelines to improve efficiency and ensure safety. In 2023, new regulations focused on simplifying trial design, especially for adaptive trials and decentralized models. These updates have shortened approval timelines by an average of 3-4 months, particularly for trials involving innovative therapies such as gene editing and personalized medicine. The FDA allocated $400 million to support the implementation of these streamlined guidelines. This initiative is expected to reduce the time to market for new drugs, improving access to therapies for patients.
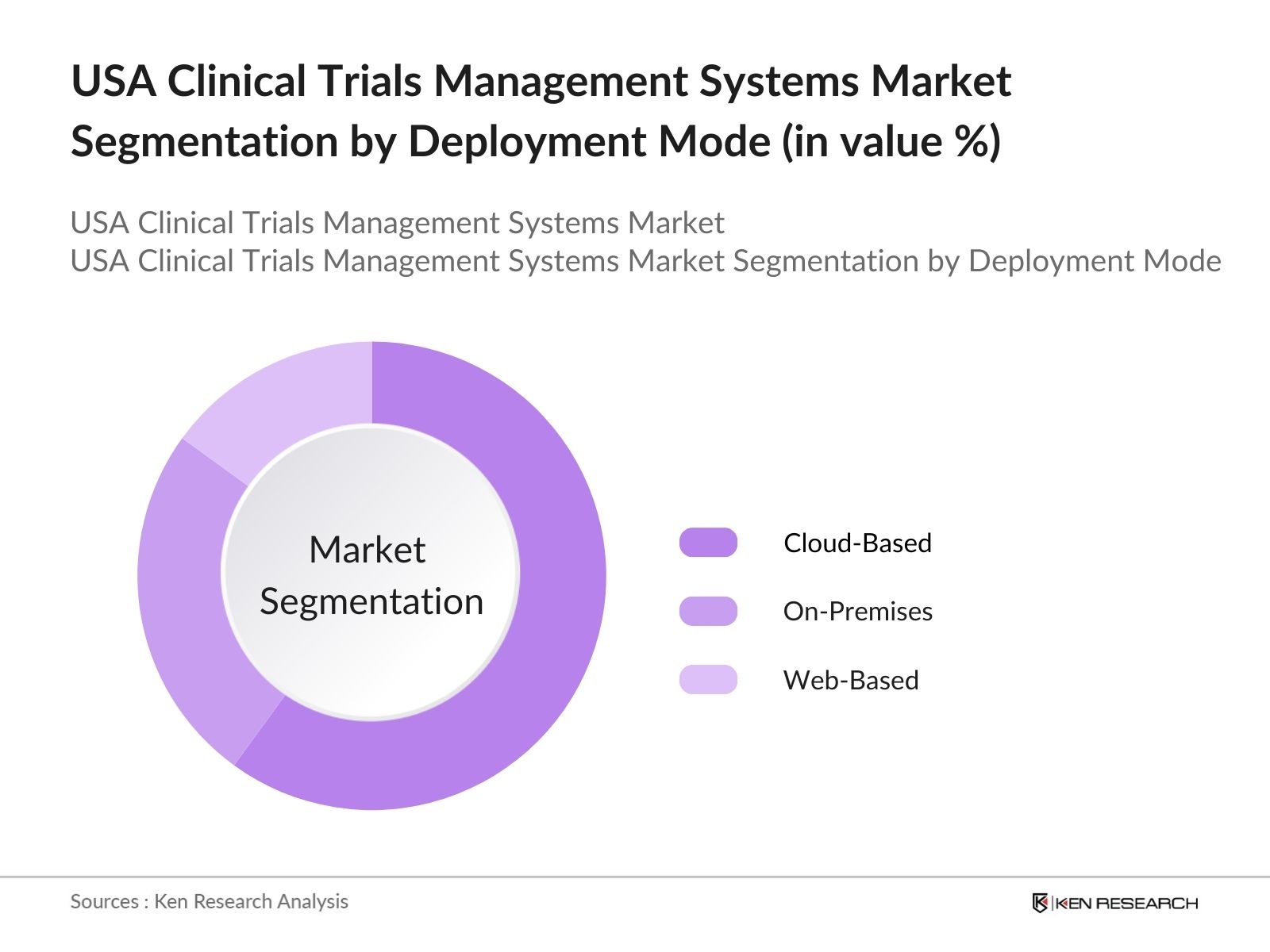
USA Clinical Trials Management Systems Market Segmentation
By Deployment Mode: The USA Clinical Trials Management Systems market is segmented by deployment mode into cloud-based, on-premises, and web-based systems. Cloud-based systems currently hold a dominant market share due to the increasing preference for flexible, scalable, and cost-effective solutions. Cloud platforms enable seamless collaboration between global research teams, secure data management, and enhanced patient recruitment processes, which are critical in modern clinical trials. Cloud solutions are expected to continue driving growth in this segment as they reduce operational costs and improve data accessibility.
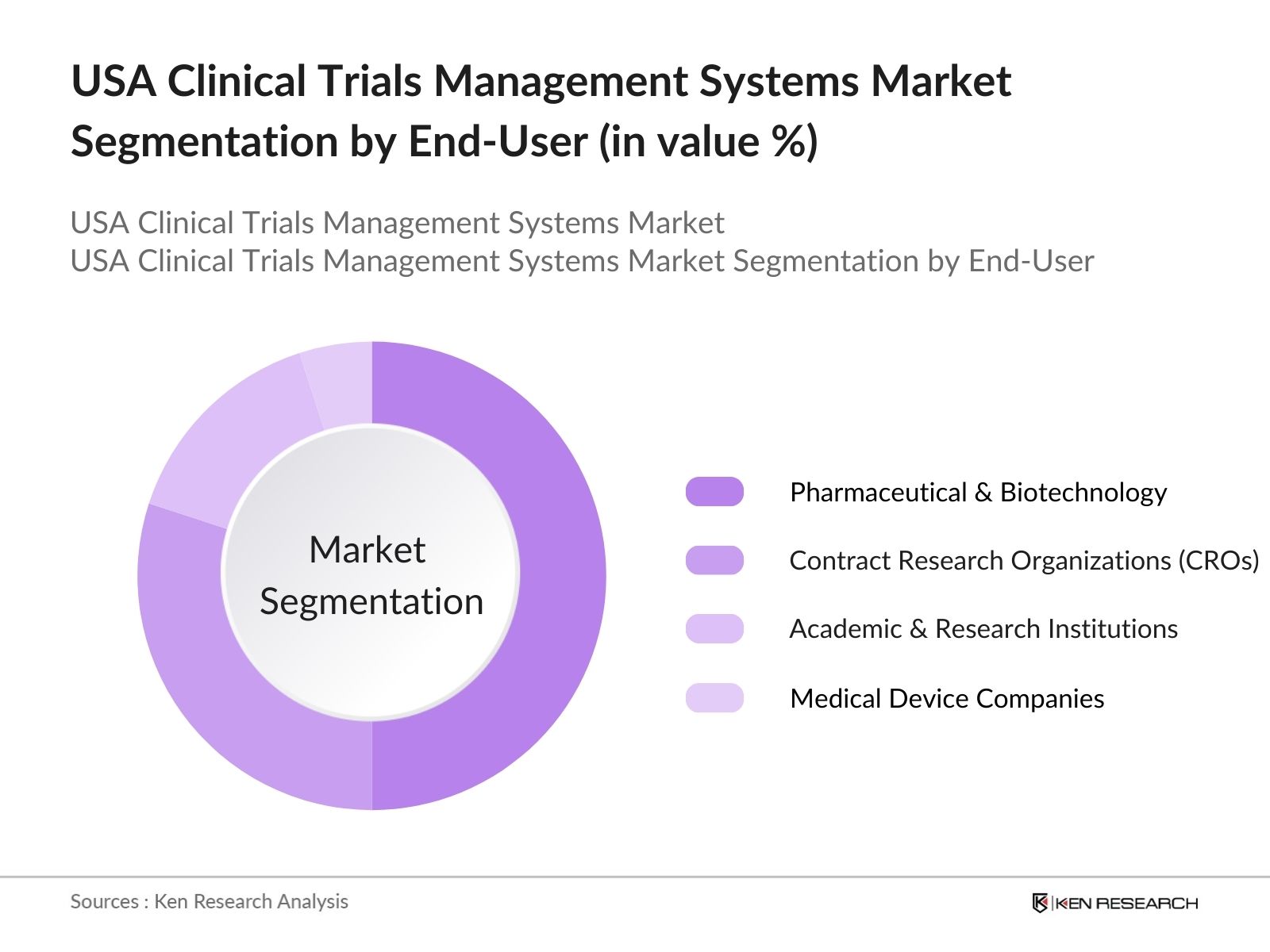
 By End User: The market is segmented by end users into pharmaceutical and biotechnology companies, contract research organizations (CROs), academic and research institutions, and medical device companies. Pharmaceutical and biotechnology companies dominate this segment due to their significant investment in new drug development and their growing reliance on clinical trials for product approvals. CROs are also a critical end-user group, benefiting from the outsourcing trends in clinical research, which drive the demand for specialized clinical trials management services.
By End User: The market is segmented by end users into pharmaceutical and biotechnology companies, contract research organizations (CROs), academic and research institutions, and medical device companies. Pharmaceutical and biotechnology companies dominate this segment due to their significant investment in new drug development and their growing reliance on clinical trials for product approvals. CROs are also a critical end-user group, benefiting from the outsourcing trends in clinical research, which drive the demand for specialized clinical trials management services.
USA Clinical Trials Management Systems Market Competitive Landscape
The USA Clinical Trials Management Systems market is dominated by a few key players who have established their presence through technological innovations and strategic partnerships. The market is competitive, with companies such as Medidata Solutions, Oracle Corporation, Parexel International, and others leading the way. These companies are investing heavily in research and development, as well as in expanding their global footprints, which includes increasing their technological capabilities for managing complex and large-scale clinical trials.
Table 3: Competitive Landscape
USA Clinical Trials Management Systems Market Analysis
Growth Drivers
- Increasing R&D Expenditure: In 2023, the U.S. spent over $680 billion on research and development, a significant portion of which was directed towards life sciences and clinical trials. Approximately $94 billion was allocated specifically to clinical research, making up 14% of the total R&D budget. The National Institutes of Health (NIH) alone contributed $45 billion to medical research, with an emphasis on clinical trials for novel therapeutics. This increase in funding underscores the heightened focus on clinical research in the pharmaceutical and biotechnology sectors.
- Technological Advancements in Clinical Trials: The integration of AI, telemedicine, and e-trials has transformed the clinical trial landscape in the U.S. By 2024, AI-enabled trials and telemedicine usage have optimized trial phases, reducing trial timelines by approximately 20%. The NIHs ongoing investments in AI-driven platforms for trials have accelerated recruitment and data processing. Over $500 million was allocated to AI in clinical research in 2023, including partnerships with private companies to develop predictive models for drug efficacy. Telemedicine platforms like Medidata have also grown, with over 4,500 clinical trials adopting e-trials, supporting the shift toward decentralized trial models.
- Rising Demand for Personalized Medicine: The rising interest in personalized medicine has resulted in a 15% increase in clinical trials targeting specific patient segments by 2024. The NIH and FDA have supported personalized clinical trial designs, focusing on genetic-based segmentation, with over 1,200 trials registered for precision therapies. The demand for patient-specific trials is projected to continue rising, supported by NIH funding of $350 million allocated to personalized medicine research in 2023.
Challenges
- High Operational Costs: The cost of clinical trials in the U.S. remains a major challenge, with the average phase III trial costing around $48 million. In 2023, total clinical trial costs for large pharmaceutical companies exceeded $50 billion. The rising operational costs stem from expensive site management, participant recruitment, and data management systems. High costs per trial phase have been a barrier to entry for smaller biotechs, limiting their capacity to compete with larger firms.
- Regulatory Hurdles: Regulatory complexities add significant time and cost to clinical trial processes in the U.S. The average approval time for new drugs by the FDA in 2023 was around 14 months, with lengthy review periods for compliance, patient safety, and ethical considerations. Companies face substantial delays in bringing drugs to market due to evolving FDA guidelines and stringent safety requirements. Furthermore, compliance costs averaged $4 billion annually for large pharmaceutical firms in 2023, driven by adherence to FDA, NIH, and international guidelines.
USA Clinical Trials Management Systems Market Future Outlook
The USA Clinical Trials Management Systems market is expected to experience significant growth over the next five years. This growth will be fueled by ongoing advancements in data management technologies, increased regulatory requirements for transparency and compliance, and the expansion of clinical trials for rare diseases and personalized medicine. The rising adoption of artificial intelligence and machine learning in clinical trials, along with the growing focus on decentralized trials, will further drive market expansion.
Market Opportunities
- Integration of AI and Big Data in Trial Processes: The integration of AI and Big Data is revolutionizing clinical trials in the U.S., with over $1.5 billion invested in 2023 to develop AI-driven solutions for trial optimization. AI technologies are being used to streamline patient recruitment, predict trial outcomes, and improve data analysis efficiency. NIH-backed projects such as the All of Us Research Program aim to leverage Big Data for personalized medicine, boosting trial precision. The adoption of AI is creating opportunities for faster, more cost-effective trials, with over 2,000 AI-powered clinical trials currently underway in the U.S.
- Decentralized Clinical Trials: Decentralized clinical trials (DCTs) have gained momentum, with more than 1,000 trials adopting virtual or hybrid models in the U.S. by 2023. This shift is driven by advances in telemedicine, wearable technologies, and e-trials. DCTs offer significant cost savings and broader patient access, reducing site management costs by nearly 30%. The FDA has supported the adoption of decentralized models, providing new guidelines to facilitate virtual trials. This growing trend presents opportunities for faster trial execution, lower costs, and improved patient retention, particularly for rare disease and rural patient populations.
Scope of the Report
|
Segment |
Sub-segment |
|
By Deployment Mode |
Web-based Cloud-based On-premises |
|
By Application |
Pharmaceutical & Biotechnology Companies Medical Device Companies Contract Research Organizations (CROs) Academic & Research Institutes |
|
By End User |
Clinical Research Sites Hospitals & Healthcare Providers Government Organizations Others |
|
By Service Type |
Project Management Data Management Monitoring Services Others |
|
By Therapeutic Area |
Oncology Cardiology Neurology Rare Diseases |
Products
Key Target Audience
Pharmaceutical and Biotechnology Companies
Medical Device Manufacturers
Contract Research Organizations (CROs)
Academic and Research Institutions
Investors and Venture Capitalist Firms
Government and Regulatory Bodies (FDA, NIH)
Clinical Research Sites
Technology Providers for Clinical Trials
Companies
Players Mentioned in the Report
Medidata Solutions
Oracle Corporation
Parexel International
BioClinica, Inc.
PPD, Inc.
Veeva Systems
ICON plc
Covance Inc.
IQVIA
PRA Health Sciences
Table of Contents
1. USA Clinical Trials Management Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (in relation to number of trials, success rates)
1.4. Market Segmentation Overview
2. USA Clinical Trials Management Market Size (In USD Mn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. USA Clinical Trials Management Market Analysis
3.1. Growth Drivers
3.1.1. Increasing R&D Expenditure (clinical trials as a % of overall R&D spending)
3.1.2. Technological Advancements in Clinical Trials (AI, e-trials, telemedicine)
3.1.3. Rising Demand for Personalized Medicine (trials based on patient segmentation)
3.1.4. Expanding Biotechnology and Pharmaceutical Sectors (new drug trials per year)
3.2. Market Challenges
3.2.1. High Operational Costs (average cost per trial phase)
3.2.2. Regulatory Hurdles (time to approval, FDA compliance complexities)
3.2.3. Participant Recruitment Difficulties (percentage of delayed trials due to recruitment)
3.2.4. Data Management and Integrity (trial data security concerns, % of trials halted due to data issues)
3.3. Opportunities
3.3.1. Integration of AI and Big Data in Trial Processes
3.3.2. Decentralized Clinical Trials (virtual and hybrid models)
3.3.3. Growth of Rare Disease Trials (orphan drug designations)
3.3.4. Outsourcing of Trials to Specialized Contract Research Organizations (CROs)
3.4. Trends
3.4.1. Increased Use of Wearable Devices for Real-Time Data Collection (wearable penetration in trials)
3.4.2. Collaborative Trials and Globalization of Clinical Research (cross-border trial expansion)
3.4.3. Growing Adoption of Real-World Evidence (RWE) and Real-Time Analytics
3.4.4. Rising Focus on Patient-Centric Trials (patient engagement metrics)
3.5. Government Regulation
3.5.1. FDA Clinical Trial Guidelines (impact of new regulations on trial timelines)
3.5.2. GDPR and Data Privacy Regulations (impact on US-EU trial collaboration)
3.5.3. Public-Private Partnerships in Clinical Research (number of PPP initiatives)
3.5.4. National Institutes of Health (NIH) Initiatives (funding allocation)
3.6. SWOT Analysis
3.7. Stake Ecosystem (influence of hospitals, CROs, biopharma)
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. USA Clinical Trials Management Market Segmentation
4.1. By Deployment Mode (In Value %)
4.1.1. Web-based
4.1.2. Cloud-based
4.1.3. On-premises
4.2. By Application (In Value %)
4.2.1. Pharmaceutical & Biotechnology Companies
4.2.2. Medical Device Companies
4.2.3. Contract Research Organizations (CROs)
4.2.4. Academic & Research Institutes
4.3. By End User (In Value %)
4.3.1. Clinical Research Sites
4.3.2. Hospitals & Healthcare Providers
4.3.3. Government Organizations
4.3.4. Others
4.4. By Service Type (In Value %)
4.4.1. Project Management
4.4.2. Data Management
4.4.3. Monitoring Services
4.4.4. Others
4.5. By Therapeutic Area (In Value %)
4.5.1. Oncology
4.5.2. Cardiology
4.5.3. Neurology
4.5.4. Rare Diseases
5. USA Clinical Trials Management Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Medidata Solutions
5.1.2. Parexel International Corporation
5.1.3. BioClinica, Inc.
5.1.4. Oracle Corporation
5.1.5. Veeva Systems
5.1.6. ICON plc
5.1.7. PPD, Inc.
5.1.8. Covance Inc.
5.1.9. IQVIA
5.1.10. PRA Health Sciences
5.2. Cross Comparison Parameters (Headquarters, Number of Trials Managed, Therapeutic Expertise, Trial Phases Covered, AI Integration, Patient Engagement, Data Security Compliance, Revenue)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. USA Clinical Trials Management Market Regulatory Framework
6.1. FDA Approval Process
6.2. Compliance with ICH Guidelines
6.3. Data Privacy Regulations (GDPR, HIPAA)
6.4. Certification Processes
7. USA Clinical Trials Management Future Market Size (In USD Mn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. USA Clinical Trials Management Future Market Segmentation
8.1. By Deployment Mode (In Value %)
8.2. By Application (In Value %)
8.3. By End User (In Value %)
8.4. By Service Type (In Value %)
8.5. By Therapeutic Area (In Value %)
9. USA Clinical Trials Management Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Patient Recruitment Optimization
9.3. Data Management Efficiency Improvement
9.4. Expansion Opportunities
Research Methodology
Step 1: Identification of Key Variables
The first step involves identifying the key factors driving the USA Clinical Trials Management Systemsmarket. This includes analyzing the key stakeholders, such as pharmaceutical companies, CROs, and regulatory agencies. We utilized a variety of secondary sources, including proprietary databases and government reports, to create a comprehensive ecosystem map of the market.
Step 2: Market Analysis and Construction
In this phase, we examined historical data on clinical trials, including the number of trials conducted, the success rate, and the geographical distribution of trials. This step focused on ensuring the accuracy of data through triangulation with multiple sources, including government publications, company reports, and expert interviews.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses were validated through consultations with industry experts, including executives from leading pharmaceutical companies and CROs. These experts provided valuable insights into operational challenges, regulatory hurdles, and market opportunities, ensuring that our data reflects the current state of the industry.
Step 4: Research Synthesis and Final Output
The final stage of our research involved synthesizing the data from various sources to create a clear, comprehensive report. This process included verifying the accuracy of our findings through primary research, including interviews and surveys of key market participants, ensuring that our analysis is robust and actionable.
Frequently Asked Questions
01. How big is the USA Clinical Trials Management Systems Market?
The USA Clinical Trials Management Systems market is valued at USD 690 million in 2023, driven by the increasing need for efficient clinical trial processes, advanced data management systems, and rising investment in R&D for personalized medicine.
02. What are the challenges in the USA Clinical Trials Management Systems Market?
The key challenges in USA Clinical Trials Management Systems market include high operational costs, complex regulatory requirements, and difficulties in patient recruitment. Additionally, data privacy concerns and the need for real-time trial monitoring are significant obstacles for companies operating in this market.
03. Who are the major players in the USA Clinical Trials Management Systems Market?
Key players in USA Clinical Trials Management Systems market include Medidata Solutions, Oracle Corporation, Parexel International, BioClinica, Inc., and PPD, Inc. These companies dominate due to their strong technological integration and established presence in the clinical trials space.
04. What are the growth drivers of the USA Clinical Trials Management Systems Market?
USA Clinical Trials Management Systems market is driven by advancements in technology, such as AI and cloud-based platforms, which streamline clinical trial processes. Additionally, the growing focus on personalized medicine and the expansion of trials into new therapeutic areas are propelling market growth.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















