
USA CRO Market Outlook to 2030
Region:North America
Author(s):Shubham
Product Code:KROD5489
November 2024
99
About the Report
USA CRO Market Overview
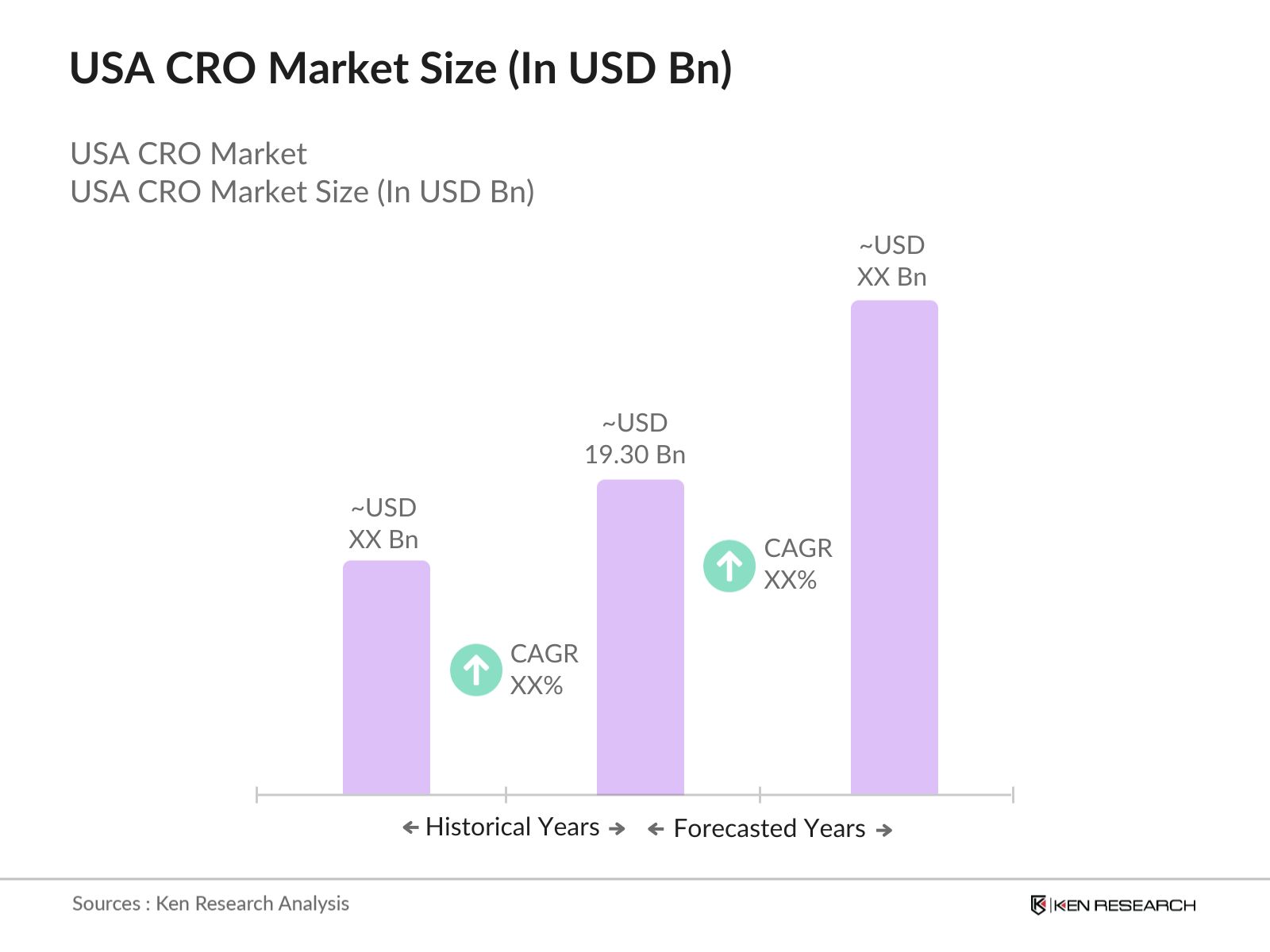
- The USA Contract Research Organization (CRO) market is valued at USD 19.30 billion, primarily driven by the increasing demand for outsourcing clinical trials and rising investments in biopharmaceutical R&D. The high cost of drug development has pushed pharmaceutical and biotechnology companies to rely on CROs for clinical trials, regulatory support, and data management. With the rise in personalized medicine and the growing complexity of clinical trials, CROs are seen as strategic partners in the research and development process, facilitating faster and more cost-effective drug discovery.
- Key cities such as Boston, San Francisco, and Raleigh-Durham play a dominant role in the USA CRO market. These cities serve as life science hubs due to their proximity to leading academic institutions, biopharmaceutical companies, and research centers. Boston, with its large concentration of biotechnology firms and research universities, leads the market, while Raleigh-Durham's Research Triangle has become a pivotal location for clinical trials. San Francisco remains crucial due to the presence of numerous venture capital firms and tech-driven healthcare startups that increasingly rely on CRO services to accelerate innovation in drug development.
- The FDA continues to evolve its guidelines to streamline clinical trials and promote faster drug approvals. In 2023, the FDA introduced over 100 new guidance documents aimed at improving trial efficiency and safety monitoring. These guidelines, including those related to adaptive trial designs and real-world evidence, are enabling CROs to take on more responsibilities in trial management and regulatory compliance, expanding their role in the pharmaceutical sector.
USA CRO Market Segmentation
- By Service Type: The market is segmented by service type into preclinical services, phase I clinical trials, phase II clinical trials, phase III clinical trials, and post-market surveillance. Preclinical services dominate the market due to their critical role in ensuring drug safety and efficacy before entering human trials. The increasing complexity of preclinical testing and the need for faster timelines have driven pharmaceutical companies to outsource these services to CROs, which have the expertise and resources to manage these early-stage research processes efficiently.
- By Therapeutic Area: The market is also segmented by therapeutic area, including oncology, cardiovascular diseases, central nervous system disorders, infectious diseases, and rare diseases. Oncology is the dominant therapeutic area, with a significant share of clinical trials focusing on cancer research. The increasing number of immuno-oncology and gene therapy trials has contributed to the growth of this segment. Oncology trials often require advanced and specialized expertise, making CROs indispensable in managing the complexities of these studies.
USA CRO Market Competitive Landscape
The USA CRO market is highly competitive, with several major players dominating through their extensive research capabilities, global presence, and strategic partnerships. These companies have built strong portfolios that cover a wide range of therapeutic areas, enabling them to meet the growing demand for outsourced clinical trials across multiple sectors. The competitive landscape is shaped by continuous innovation in clinical trial methodologies and the strategic consolidation of smaller players by larger CROs.
USA CRO Market Analysis
Growth Drivers
- Rising Outsourcing in Clinical Trials: The USA Contract Research Organization (CRO) market is seeing significant growth due to an increase in clinical trial outsourcing. Pharmaceutical companies are increasingly turning to CROs to manage the complexities of clinical trials. According to a report by the National Institutes of Health, globally over 22,000 clinical trials were initiated in 2023, a notable rise from previous years due to outsourcing trends. This has been driven by the high operational costs of in-house research and stringent regulations, making outsourcing more cost-effective for companies. Clinical trials are projected to require even more third-party services as demand for new drug approvals surges.
- Increased Biopharmaceutical R&D: The USA biopharmaceutical sector invested over USD 141 billion in R&D in 2022, driven by the rising demand for innovative treatments and therapies. This surge in research investment is propelling the growth of CRO services as biopharmaceutical companies increasingly rely on these organizations for clinical trial management and drug development processes. The focus on therapies for rare diseases and complex biologics has also pushed the need for advanced clinical research expertise, which CROs are well-positioned to provide.
- Favorable Regulatory Landscape: Favorable regulations set by the U.S. Food and Drug Administration (FDA) have created a conducive environment for clinical trials in the country. In 2023, the FDA approved over 60 new drugs, supported by streamlined trial processes and adaptive regulatory frameworks that facilitate faster drug development. The 21st Century Cures Act, along with continued federal funding, is promoting accelerated trial protocols, which allows CROs to play an integral role in handling the compliance and execution of these trials.
Challenges
- Complex Regulatory Requirements: While the U.S. regulatory environment is generally supportive of clinical research, navigating the ever-changing regulatory landscape remains a significant challenge for CROs. The FDA's regulatory guidelines are intricate, requiring CROs to continuously adapt to new rules, approvals, and compliance measures. These guidelines are frequently updated, which increases the operational complexity for CROs, especially smaller firms managing specialized clinical trials. Compliance demands can result in longer timelines and additional administrative overheads, necessitating robust internal processes.
- High Cost of Clinical Trials: The financial burden of conducting clinical trials in the U.S. remains a significant challenge for many sponsors, particularly small and mid-sized biopharmaceutical companies. Expenses associated with patient recruitment, trial management, and compliance with stringent regulatory standards continue to escalate. Long trial durations further add to these costs, making it difficult for some companies to fund the necessary stages of research and development. CROs play a critical role in mitigating these expenses by offering specialized services that optimize trial processes, but the overall high cost remains a barrier that affects the broader clinical research industry.
USA CRO Market Future Outlook
The USA CRO market is expected to witness significant growth, driven by ongoing advancements in clinical trial technologies, the rising demand for precision medicine, and the increasing focus on rare diseases. As pharmaceutical companies continue to outsource research to reduce costs and streamline operations, CROs will play an even more critical role in the drug development ecosystem. Additionally, the expansion of digital health technologies and decentralized clinical trials is expected to open new avenues for growth in the CRO market.
Future Market Opportunities
- Expansion of Personalized Medicine: The growing emphasis on personalized medicine is significantly increasing the demand for specialized clinical trials, creating substantial opportunities for CROs to expand their service offerings. With a focus on tailoring treatments to individual patient profiles based on genetic, environmental, and lifestyle factors, pharmaceutical companies are heavily investing in precision medicine. By 2023, over 34% of new drug approvals in the U.S. were personalized treatments, a clear indication of this shift. As a result, CROs with expertise in genomics and biomarkers are becoming critical partners for pharmaceutical companies, further expanding their involvement in cutting-edge biopharmaceutical development.
- Increasing Focus on Rare Diseases: Rare diseases impact approximately 30 million people in the U.S., creating an urgent need for new therapies and treatments. As of 2023, more than 7,000 rare diseases have been identified, yet there are only 500 FDA-approved treatments available, highlighting the significant unmet medical need in this area. The Orphan Drug Act continues to provide financial incentives for pharmaceutical companies to focus on these conditions, offering an expansive opportunity for CROs to manage specialized clinical trials. This increased attention to rare diseases ensures that CROs are at the forefront of developing therapies for these underserved patient populations.
Scope of the Report
|
Service Type |
Preclinical Services Phase I Clinical Trials Phase II Clinical Trials Phase III Clinical Trials Post-Market Surveillance |
|
Therapeutic Area |
Oncology Cardiovascular Diseases Central Nervous System Disorders Infectious Diseases Rare Diseases |
|
End-User |
Pharmaceutical Companies Biotechnology Firms Medical Device Manufacturers Government Research Institutes Academic Institutes |
|
Delivery Model |
Full-Service Model Functional Service Provider (FSP) Model Hybrid Models |
|
Region |
North East Mid-West West Coast Southern States |
Products
Key Target Audience
Pharmaceutical Companies
Biotechnology Firms
Medical Device Manufacturers
Academic Research Institutions
Government and Regulatory Bodies (FDA, NIH)
Investors and Venture Capitalist Firms
Banks and Financial Institutions
Healthcare Providers and Hospitals
Companies
Players Mentioned in the Report
IQVIA
Labcorp Drug Development
Syneos Health
PPD (Thermo Fisher)
ICON Plc
PRA Health Sciences
Medpace
Parexel
Charles River Laboratories
Covance
WuXi AppTec
KCR
Worldwide Clinical Trials
Envigo
Clinipace
Table of Contents
1. USA CRO Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate (Revenue, Clinical Trials Volume)
1.4. Market Segmentation Overview
USA CRO Market Size (in USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
USA CRO Market Analysis
3.1. Growth Drivers
3.1.1. Rising Outsourcing in Clinical Trials
3.1.2. Increased Biopharmaceutical R&D
3.1.3. Technological Advancements (AI, Big Data)
3.1.4. Favorable Regulatory Landscape
3.2. Market Challenges
3.2.1. Complex Regulatory Requirements
3.2.2. High Cost of Clinical Trials
3.2.3. Skilled Workforce Shortages
3.3. Opportunities
3.3.1. Expansion of Personalized Medicine
3.3.2. Increasing Focus on Rare Diseases
3.3.3. Partnerships with Academic Institutions
3.4. Trends
3.4.1. Decentralized Clinical Trials
3.4.2. Increased Use of AI in Drug Discovery
3.4.3. Shift Toward Precision Medicine
3.5. Government Regulation
3.5.1. FDA Guidelines on Clinical Trials
3.5.2. Data Privacy Regulations (HIPAA, GDPR Compliance)
3.5.3. Federal Funding Initiatives
USA CRO Market Segmentation
4.1. By Service Type (in Value %)
4.1.1. Preclinical Services
4.1.2. Phase I Clinical Trials
4.1.3. Phase II Clinical Trials
4.1.4. Phase III Clinical Trials
4.1.5. Post-Market Surveillance
4.2. By Therapeutic Area (in Value %)
4.2.1. Oncology
4.2.2. Cardiovascular Diseases
4.2.3. Central Nervous System Disorders
4.2.4. Infectious Diseases
4.2.5. Rare Diseases
4.3. By End-User (in Value %)
4.3.1. Pharmaceutical Companies
4.3.2. Biotechnology Firms
4.3.3. Medical Device Manufacturers
4.3.4. Government Research Institutes
4.3.5. Academic Institutes
4.4. By Region (in Value %)
4.4.1. North East
4.4.2. Mid-West
4.4.3. West Coast
4.4.4. Southern States
4.5. By Delivery Model (in Value %)
4.5.1. Full-Service Model
4.5.2. Functional Service Provider (FSP) Model
4.5.3. Hybrid Models
USA CRO Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. IQVIA
5.1.2. Labcorp Drug Development
5.1.3. Syneos Health
5.1.4. PPD (Thermo Fisher)
5.1.5. ICON Plc
5.1.6. PRA Health Sciences
5.1.7. Medpace
5.1.8. Parexel
5.1.9. Charles River Laboratories
5.1.10. Covance
5.1.11. WuXi AppTec
5.1.12. KCR
5.1.13. Worldwide Clinical Trials
5.1.14. Envigo
5.1.15. Clinipace
5.2. Cross Comparison Parameters (Revenue, Global Presence, Number of Clinical Trials, Therapeutic Areas of Focus, No. of Employees, R&D Spend, Strategic Partnerships, Acquisition History)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Government and Private Equity Funding
USA CRO Market Regulatory Framework
6.1. FDA Guidelines
6.2. Compliance with ICH-GCP
6.3. Certification Processes
6.4. Data Integrity and Security Requirements
USA CRO Future Market Size (in USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
USA CRO Future Market Segmentation
8.1. By Service Type (in Value %)
8.2. By Therapeutic Area (in Value %)
8.3. By End-User (in Value %)
8.4. By Delivery Model (in Value %)
8.5. By Region (in Value %)
USA CRO Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Key Partnership Recommendations
9.3. White Space Opportunity Analysis
9.4. Clinical Trial Success Metrics
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The initial phase involved constructing an ecosystem map of the USA CRO market. This step utilized a combination of secondary research and proprietary databases to gather industry-level information. The main objective was to identify the critical variables driving market dynamics, including service type, therapeutic focus, and regulatory environment.
Step 2: Market Analysis and Construction
This phase focused on compiling and analyzing historical data from the USA CRO market, covering aspects such as market penetration, clinical trial volumes, and revenue generation. The goal was to assess service provider performance and the overall impact of CRO services on drug development timelines.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses were formulated and tested through consultations with industry experts via CATIs (Computer-Assisted Telephone Interviews). These discussions provided valuable insights into CRO operations, pricing strategies, and emerging trends in the market.
Step 4: Research Synthesis and Final Output
The final phase involved engaging directly with CRO service providers and pharmaceutical firms to validate the data. This bottom-up approach ensured the accuracy and comprehensiveness of the market insights presented in the report.
Frequently Asked Questions
01. How big is the USA CRO Market?
The USA CRO market is valued at USD 19.30 billion and is driven by the increasing outsourcing of clinical trials and rising pharmaceutical R&D investments.
02. What are the challenges in the USA CRO Market?
Challenges in the USA CRO market include regulatory complexities, high clinical trial costs, and the need for skilled professionals in niche therapeutic areas.
03. Who are the major players in the USA CRO Market?
Major players in the USA CRO market include IQVIA, Labcorp Drug Development, Syneos Health, ICON Plc, and PPD (Thermo Fisher).
04. What are the growth drivers of the USA CRO Market?
Key drivers in the USA CRO market include rising demand for outsourcing, advancements in precision medicine, and innovations in data management and clinical trial technologies.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















