
USA External Ventricular Drain Market Outlook to 2030
Region:North America
Author(s):Yogita Sahu
Product Code:KROD5289
November 2024
94
About the Report
USA External Ventricular Drain Market Overview
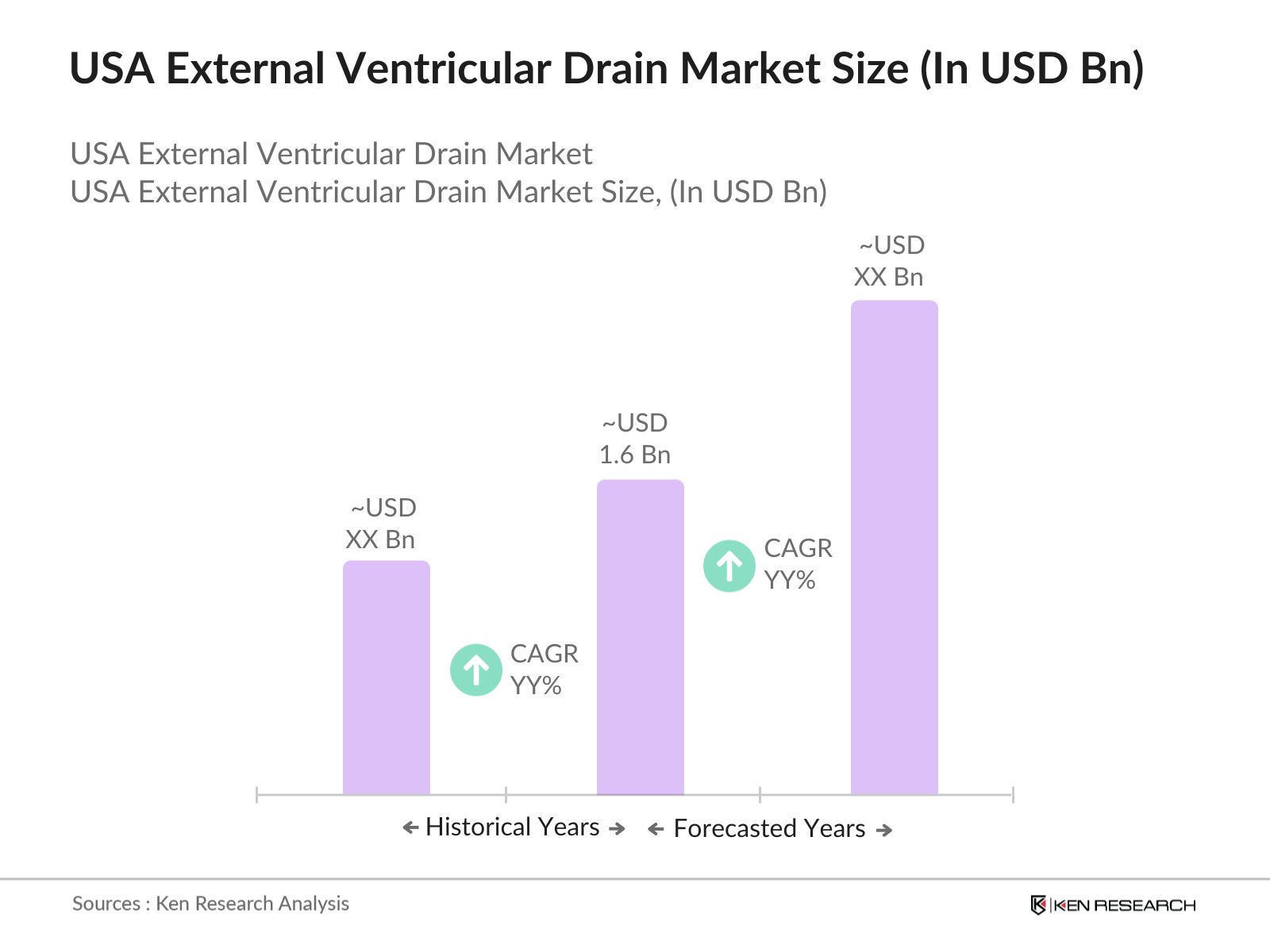
- The USA External Ventricular Drain (EVD) market was valued at USD 1.6 billion. This market is primarily driven by the rising incidence of neurological disorders such as hydrocephalus, traumatic brain injuries, and strokes. The growing aging population and increasing demand for advanced neurosurgical interventions in the U.S. healthcare system are significant contributors to market growth.
- The market, with cities such as New York, Los Angeles, and Chicago leading the way. These regions are home to top-tier healthcare institutions, including specialized neurosurgical centers, driving higher adoption of EVD systems. The dominance of these cities can be attributed to the availability of skilled neurosurgeons, advanced healthcare infrastructure, and investments in healthcare technology, positioning the U.S. as a global leader in neurological care.
- The U.S. government's continued expansion of Medicare and Medicaid programs, which now covers over 82 million people in 2024, has increased access to healthcare services, including critical neurological procedures that involve the use of EVDs.
USA External Ventricular Drain Market Segmentation
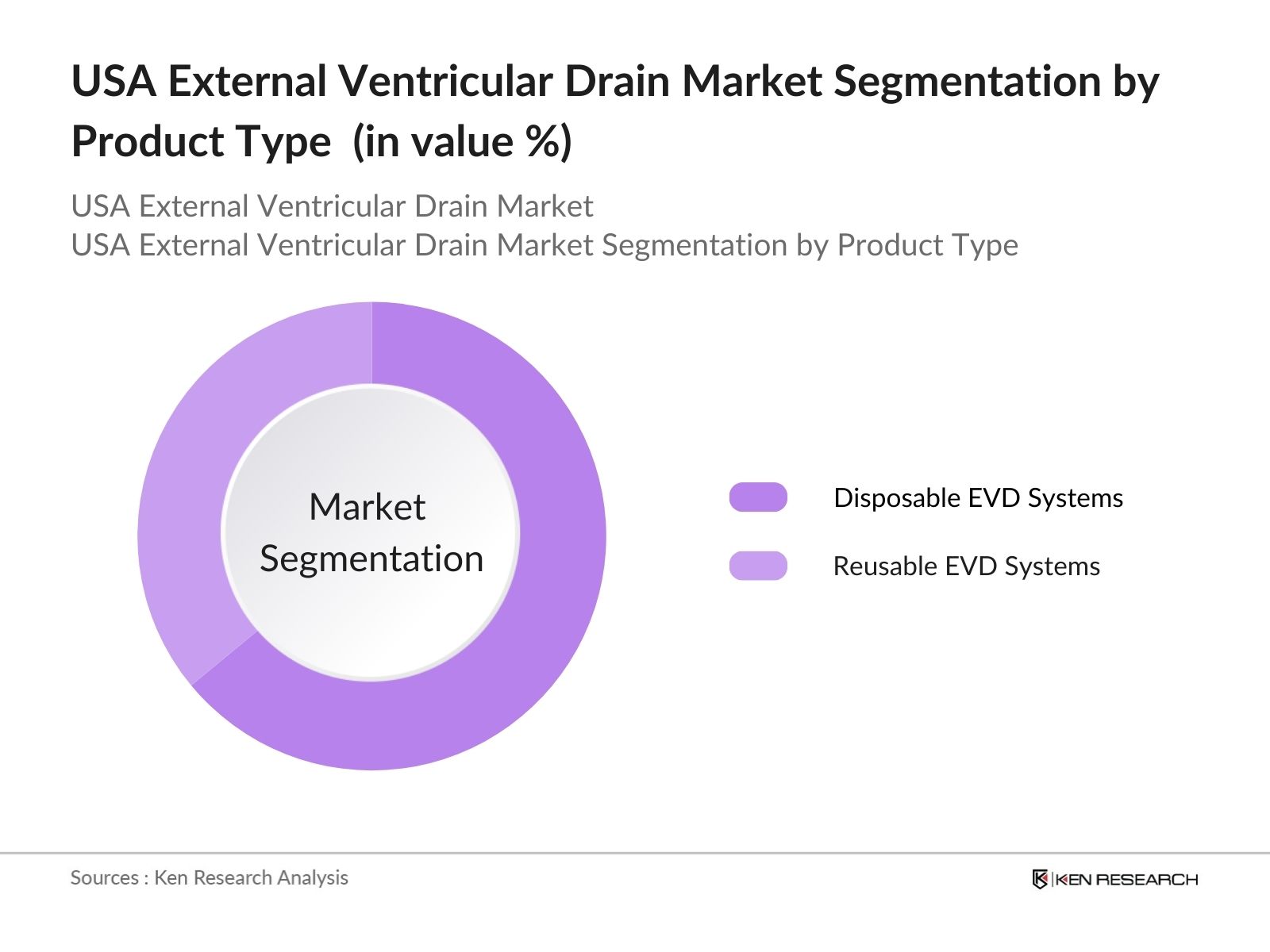
By Product Type: The market is segmented by product type into Disposable EVD Systems and Reusable EVD Systems. Disposable EVD Systems have a dominant market share due to their increasing adoption in hospitals and surgical centers. This dominance stems from the convenience and reduced risk of infections associated with disposable systems. Healthcare facilities are prioritizing disposable systems to prevent hospital-acquired infections, improve patient outcomes, and comply with stringent FDA and CDC guidelines regarding infection control.
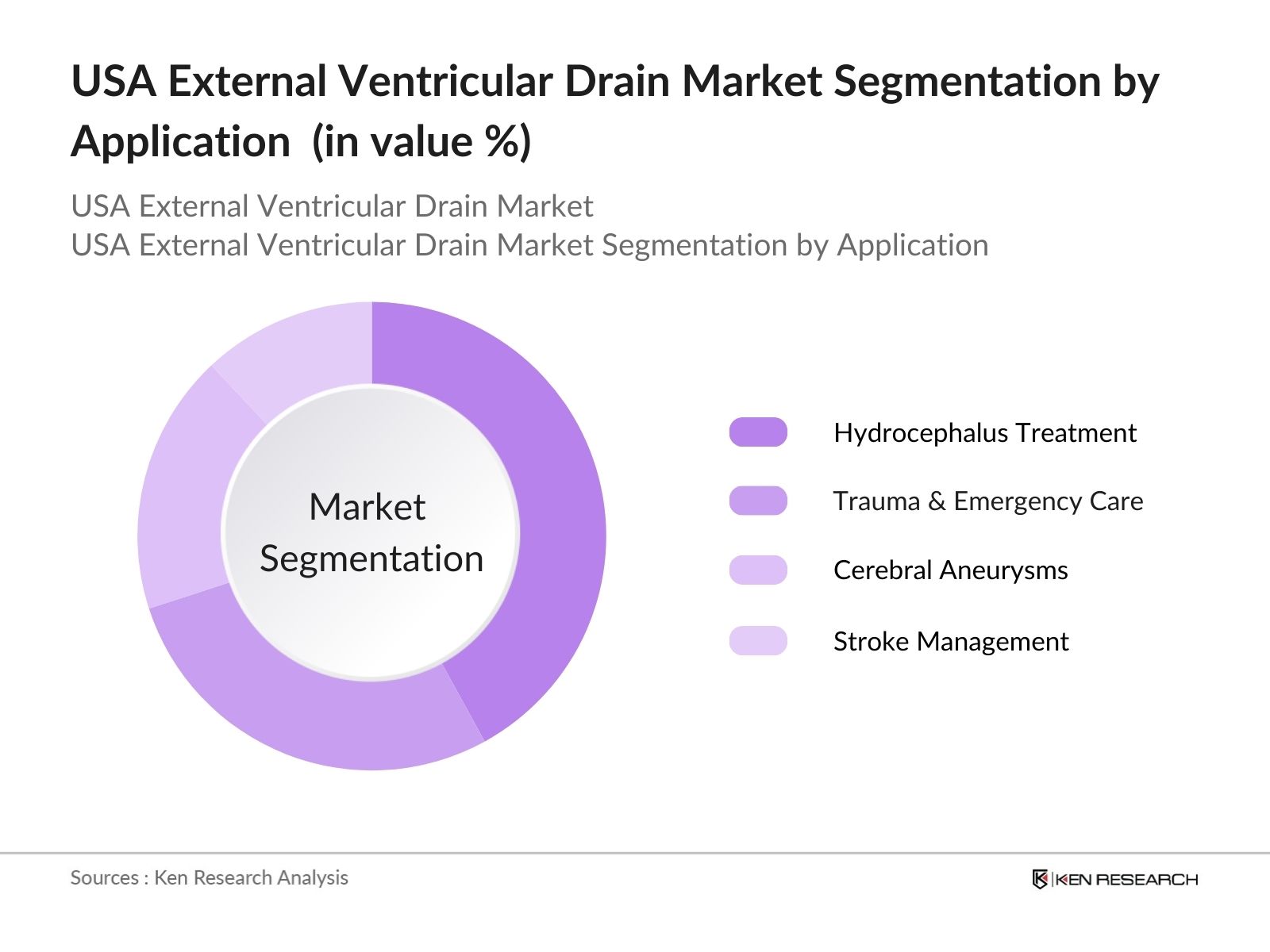
By Application: The market is also segmented by application into Trauma & Emergency Care, Cerebral Aneurysms, Stroke Management, and Hydrocephalus Treatment. Hydrocephalus Treatment leads this segmentation, with a significant market share. Hydrocephalus, which is prevalent among both pediatric and geriatric populations, drives the demand for EVD systems. Early diagnosis and the rise of minimally invasive neurosurgical techniques have contributed to this segment's growth, supported by increased healthcare funding and advanced medical devices tailored for treating hydrocephalus.
USA External Ventricular Drain Market Competitive Landscape
The market is consolidated, with a few key players controlling significant market shares. Companies like Medtronic PLC and Integra Lifesciences Holdings Corporation lead the market due to their robust product portfolios, continuous product innovations, and strong distribution networks. These companies have made substantial investments in research and development to introduce next-generation EVD systems, further driving market growth.
|
Company |
Established Year |
Headquarters |
No. of Patents Filed |
R&D Expenditure (%) |
Market Share (%) |
Key Product Portfolio |
Partnerships/Collaborations |
FDA Approvals |
|
Medtronic PLC |
1949 |
Dublin, Ireland |
||||||
|
Integra Lifesciences Holdings Corp |
1989 |
Princeton, NJ, USA |
||||||
|
B. Braun Melsungen AG |
1839 |
Melsungen, Germany |
||||||
|
Johnson & Johnson (DePuy Synthes) |
1886 |
New Brunswick, NJ, USA |
||||||
|
Sophysa |
1976 |
Orsay, France |
USA External Ventricular Drain Market Analysis
Market Growth Drivers
- Increasing Prevalence of Neurological Disorders: The USA has witnessed a rise in neurological disorders, particularly traumatic brain injuries (TBIs), which is a major factor driving the demand for external ventricular drains (EVDs). In 2024, the CDC reported over 2.8 million annual emergency department visits due to TBI-related complications, leading to an increased requirement for EVD procedures to manage intracranial pressure.
- Government Healthcare Spending: U.S. government expenditure on healthcare continues to rise, with the Congressional Budget Office estimating federal health care spending to exceed $1.4 trillion in 2024. This increase in funding for hospitals and medical centers is anticipated to facilitate the procurement and use of EVDs in more medical facilities across the country.
- Increasing Number of Neurosurgeries: The increasing availability of specialized neurosurgical centers in the USA has driven up the number of brain surgeries, many of which require EVDs for postoperative care. According to the American Association of Neurological Surgeons, around 100,000 hydrocephalus-related surgeries occur annually in the USA, many of which involve EVD placement.
Market Challenges
- Regulatory Approval Delays: The U.S. FDAs stringent regulations on medical devices have led to delays in the approval and adoption of newer, advanced EVD technologies. In 2024, manufacturers faced an average delay of 18-24 months for the approval of EVD-related products, impacting market growth.
- Risk of EVD-Associated Infections: One of the major challenges in the EVD market is the risk of device-associated infections. Studies from 2024 show that about 10,000 nosocomial infections occur annually in the U.S., leading to extended hospital stays and additional healthcare costs, raising concerns about widespread EVD usage.
USA External Ventricular Drain Market Future Outlook
Over the next five years, the USA External Ventricular Drain industry is expected to experience sustained growth driven by continuous advancements in EVD technology, such as the development of antimicrobial and infection-resistant materials. The market's expansion will also be fueled by rising demand for neurosurgical interventions due to the growing elderly population and the increased prevalence of conditions like hydrocephalus and traumatic brain injuries.
Future Market Opportunities
- Rise in Demand for Minimally Invasive EVD Systems: Over the next five years, the demand for minimally invasive EVD systems is expected to grow in the U.S. market, driven by the push for shorter recovery times and reduced infection risks. Leading manufacturers will likely develop systems with enhanced biocompatibility and infection control measures.
- Increased Adoption of Telemonitoring in EVD Systems: By 2029, telemonitoring capabilities in EVD systems will become more commonplace in the U.S. healthcare system. This trend will allow neurosurgeons to remotely monitor patient conditions, reducing the need for extended hospital stays and improving patient outcomes.
Scope of the Report
|
By Product Type |
Disposable EVD Systems Reusable EVD Systems |
|
By Application |
Trauma & Emergency Care Cerebral Aneurysms Stroke Management Hydrocephalus Treatment |
|
By End-User |
Hospitals Specialized Neurosurgical Centers Academic & Research Institutes |
|
By Material Type |
Silicone-based EVD Polyurethane-based EVD Antimicrobial-coated EVD |
|
By Region |
North West East South |
Products
Key Target Audience Organizations and Entities Who Can Benefit by Subscribing This Report:
Banks and Financial Institution
Medical Device Manufacturers
Private Equity Firms
Government and Regulatory Bodies (FDA, CDC)
Investments and Venture Capitalist Firms
Health Insurance Companies
Private Healthcare Providers
Telehealth and Remote Monitoring Providers
Companies
Players Mentioned in the Report:
Medtronic PLC
Integra Lifesciences Holdings Corporation
Johnson & Johnson (DePuy Synthes)
B. Braun Melsungen AG
Sophysa
Moller Medical GmbH
Natus Medical Incorporated
Raumedic AG
Edwards Lifesciences Corporation
Teleflex Inc.
BeckerSmith Medical
Spiegelberg GmbH & Co. KG
Smiths Medical
Nihon Kohden Corporatio
Tokibo Co., Ltd.
Table of Contents
USA External Ventricular Drain Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
USA External Ventricular Drain Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
USA External Ventricular Drain Market Analysis
3.1. Growth Drivers
3.1.1. Rising Incidence of Neurological Disorders (Hospital Admission Rates, Diagnosis Rates)
3.1.2. Increasing Adoption of Minimally Invasive Procedures (Surgical Innovation Metrics)
3.1.3. Technological Advancements in Drainage Systems (Patent Filings, Product Launches)
3.1.4. Rising Geriatric Population (Age Demographics, Chronic Disease Incidences)
3.2. Market Challenges
3.2.1. High Costs of Procedures (Healthcare Expenditure Analysis)
3.2.2. Lack of Skilled Neurosurgeons (Healthcare Workforce Availability)
3.2.3. Risk of Infections (Hospital-acquired Infection Rates, Complication Statistics)
3.3. Opportunities
3.3.1. Innovations in Infection-Resistant EVD Products (R&D Investments, FDA Approvals)
3.3.2. Increasing Adoption in Developing Regions (Healthcare Infrastructure Growth)
3.3.3. Government Initiatives Supporting Neurosurgical Interventions (Healthcare Policy Reforms)
3.4. Trends
3.4.1. Development of Antimicrobial EVD Systems (Innovation Pipeline, Patent Analysis)
3.4.2. Integration with Smart Monitoring Systems (IOT Applications in Healthcare)
3.4.3. Customization and Personalization of EVD Procedures (Patient-Specific Treatment Plans)
3.5. Government Regulation
3.5.1. FDA Regulations on EVD Devices (Approval Process, Compliance Requirements)
3.5.2. Health and Safety Guidelines (CDC, WHO)
3.5.3. Government Reimbursement Programs (Medicare, Medicaid)
3.6. SWOT Analysis
3.6.1. Strengths
3.6.2. Weaknesses
3.6.3. Opportunities
3.6.4. Threats
3.7. Stake Ecosystem (Market Collaborations, Supply Chain Analysis)
3.8. Porters Five Forces (Competitive Landscape)
3.9. Competition Ecosystem (Key Market Players)
USA External Ventricular Drain Market Segmentation
4.1. By Product Type (In Value %)
4.1.1. Disposable EVD Systems
4.1.2. Reusable EVD Systems
4.2. By Application (In Value %)
4.2.1. Trauma & Emergency Care
4.2.2. Cerebral Aneurysms
4.2.3. Stroke Management
4.2.4. Hydrocephalus Treatment
4.3. By End-User (In Value %)
4.3.1. Hospitals
4.3.2. Specialized Neurosurgical Centers
4.3.3. Academic & Research Institutes
4.4. By Material Type (In Value %)
4.4.1. Silicone-based EVD
4.4.2. Polyurethane-based EVD
4.4.3. Antimicrobial-coated EVD
4.5. By Region (In Value %)
4.5.1. North
4.5.2. West
4.5.3. East
4.5.4. South
USA External Ventricular Drain Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Medtronic PLC
5.1.2. Integra Lifesciences Holdings Corporation
5.1.3. Johnson & Johnson (DePuy Synthes)
5.1.4. B. Braun Melsungen AG
5.1.5. Spiegelberg GmbH & Co. KG
5.1.6. Sophysa
5.1.7. Moller Medical GmbH
5.1.8. Natus Medical Incorporated
5.1.9. Raumedic AG
5.1.10. Tokibo Co., Ltd.
5.1.11. Edwards Lifesciences Corporation
5.1.12. Teleflex Inc.
5.1.13. BeckerSmith Medical
5.1.14. Smiths Medical
5.1.15. Nihon Kohden Corporation
5.2. Cross Comparison Parameters (Revenue, Headquarters Location, No. of Patents Filed, Product Portfolio, Research & Development Spend, Employee Count, Market Share, Partnerships)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers And Acquisitions
5.6. Investment Analysis
5.7. Government Grants
5.8. Private Equity Investments
USA External Ventricular Drain Market Regulatory Framework
6.1. FDA Approval Process for EVD Systems
6.2. Compliance with International Safety Standards (ISO, CE Mark)
6.3. Reimbursement Policies
USA External Ventricular Drain Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth (Technological Advancements, Expanding Applications in Pediatric Care)
USA External Ventricular Drain Future Market Segmentation
8.1. By Product Type
8.2. By Application
8.3. By End-User
8.4. By Material Type
8.5. By Region
USA External Ventricular Drain Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Marketing Initiatives
9.3. White Space Opportunity Analysis
Disclaimer Contact UsResearch Methodology
Step 1: Identification of Key Variables
The first step of the research process involves constructing an ecosystem map of all major stakeholders in the USA External Ventricular Drain market. This is supported by extensive desk research and analysis of secondary databases to identify critical market dynamics, including major product types, applications, and end-users.
Step 2: Market Analysis and Construction
In this phase, we analyzed historical data related to the USA EVD market. Market penetration rates, demand for different EVD systems, and healthcare infrastructure were evaluated. Revenue generation was also assessed based on service quality statistics and product performance data.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses were formulated and validated through expert consultations, conducted via telephone interviews with key industry players such as manufacturers, distributors, and healthcare professionals. This helped to verify market data and align the findings with industry trends.
Step 4: Research Synthesis and Final Output
Finally, primary data collection was combined with detailed interactions with healthcare professionals and neurosurgical experts to verify the findings. This bottom-up approach ensures that the market data is accurate and reliable for businesses seeking to understand the current market landscape.
Frequently Asked Questions
01. How big is the USA External Ventricular Drain market?
The USA External Ventricular Drain market is valued at USD 1.6 billion, driven by rising neurological conditions and increasing adoption of advanced drainage systems in healthcare institutions.
02. What are the challenges in the USA External Ventricular Drain market?
Challenges in the USA External Ventricular Drain market include high costs associated with EVD systems, lack of skilled neurosurgeons, and risks of hospital-acquired infections despite advancements in product designs.
03. Who are the major players in the USA External Ventricular Drain market?
Key players in the USA External Ventricular Drain market include Medtronic PLC, Integra Lifesciences Holdings Corporation, Johnson & Johnson (DePuy Synthes), B. Braun Melsungen AG, and Sophysa. These companies dominate due to their strong product portfolios and significant R&D investments.
04. What are the growth drivers of the USA External Ventricular Drain market?
Growth drivers in the USA External Ventricular Drain market include the increasing geriatric population, rising incidence of hydrocephalus and traumatic brain injuries, and advancements in infection-resistant EVD systems.
05. What are the market opportunities for new entrants in the USA External Ventricular Drain market?
Opportunities for new entrants include innovation in antimicrobial coatings, partnerships with healthcare institutions for product trials, and leveraging government initiatives to support advanced healthcare technologies.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















