
USA Gene Therapy Market Outlook to 2030
Region:North America
Author(s):Sanjana
Product Code:KROD3441
October 2024
86
About the Report
USA Gene Therapy Market Overview
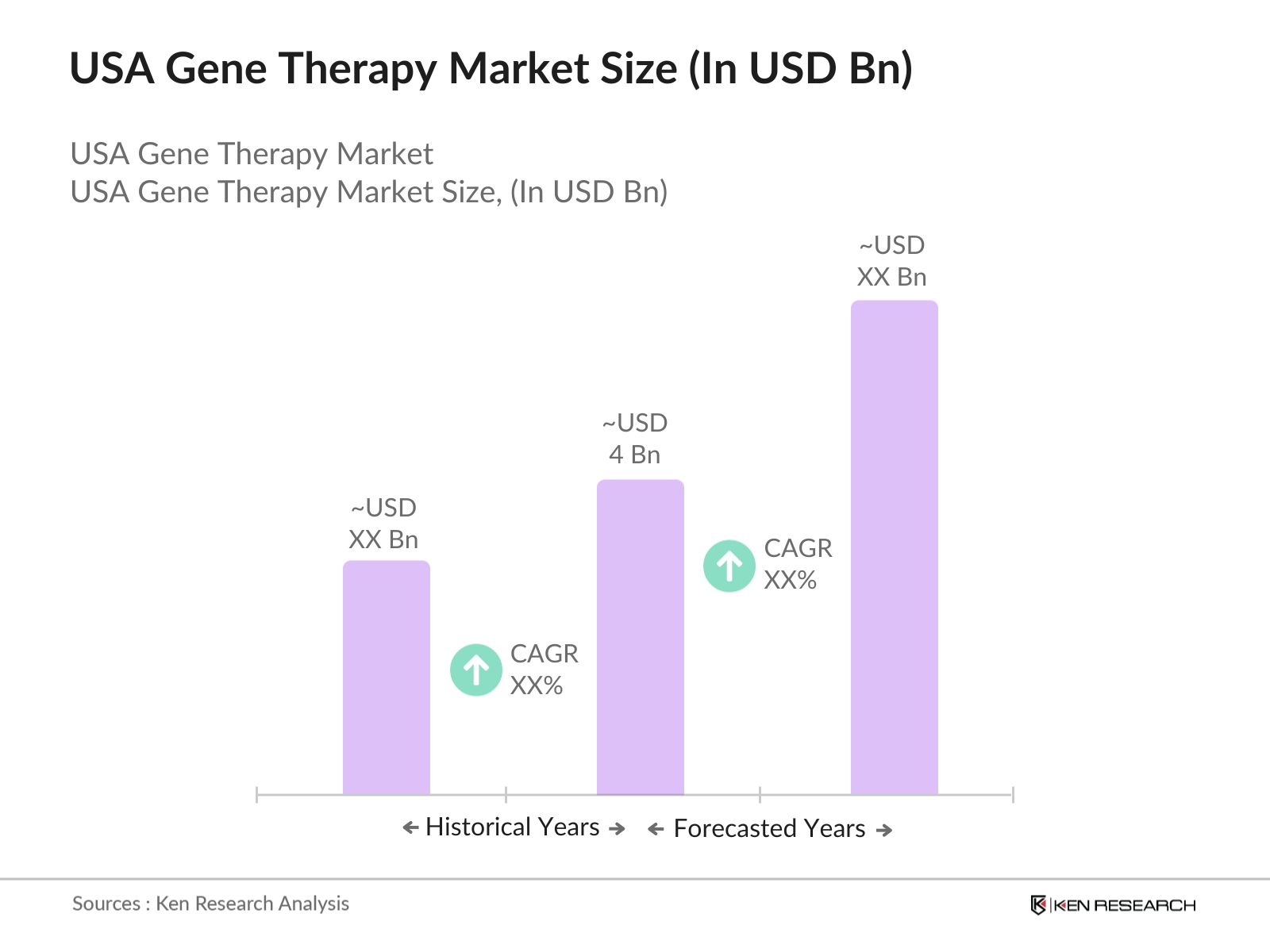
- The USA Gene Therapy market is valued at USD 4 billion, driven by the rapid growth in research and development activities, increased approval rates for gene therapy products, and the rising prevalence of genetic disorders. These factors have collectively contributed to an increase in demand for innovative treatments, particularly in oncology and rare diseases. The governments support through regulatory frameworks like the FDAs accelerated approval pathways also fuels the markets steady growth.
- The United States, especially cities like Boston and San Francisco, dominates the gene therapy market due to their highly developed biotechnology clusters, availability of skilled professionals, and significant investment in life sciences research. These cities are home to leading gene therapy companies, prestigious academic institutions, and clinical research organizations that drive advancements in the sector. Additionally, the collaborative ecosystem in these regions fosters innovation and enables faster clinical trials and regulatory approval processes.
- The FDAs regulatory pathways for gene therapy, including the Breakthrough Therapy and Regenerative Medicine Advanced Therapy (RMAT) designations, have accelerated approvals. By 2023, the FDA granted over 30 RMAT designations, underscoring the regulatory emphasis on gene therapies. This framework facilitates faster market access while maintaining safety standards.
USA Gene Therapy Market Segmentation
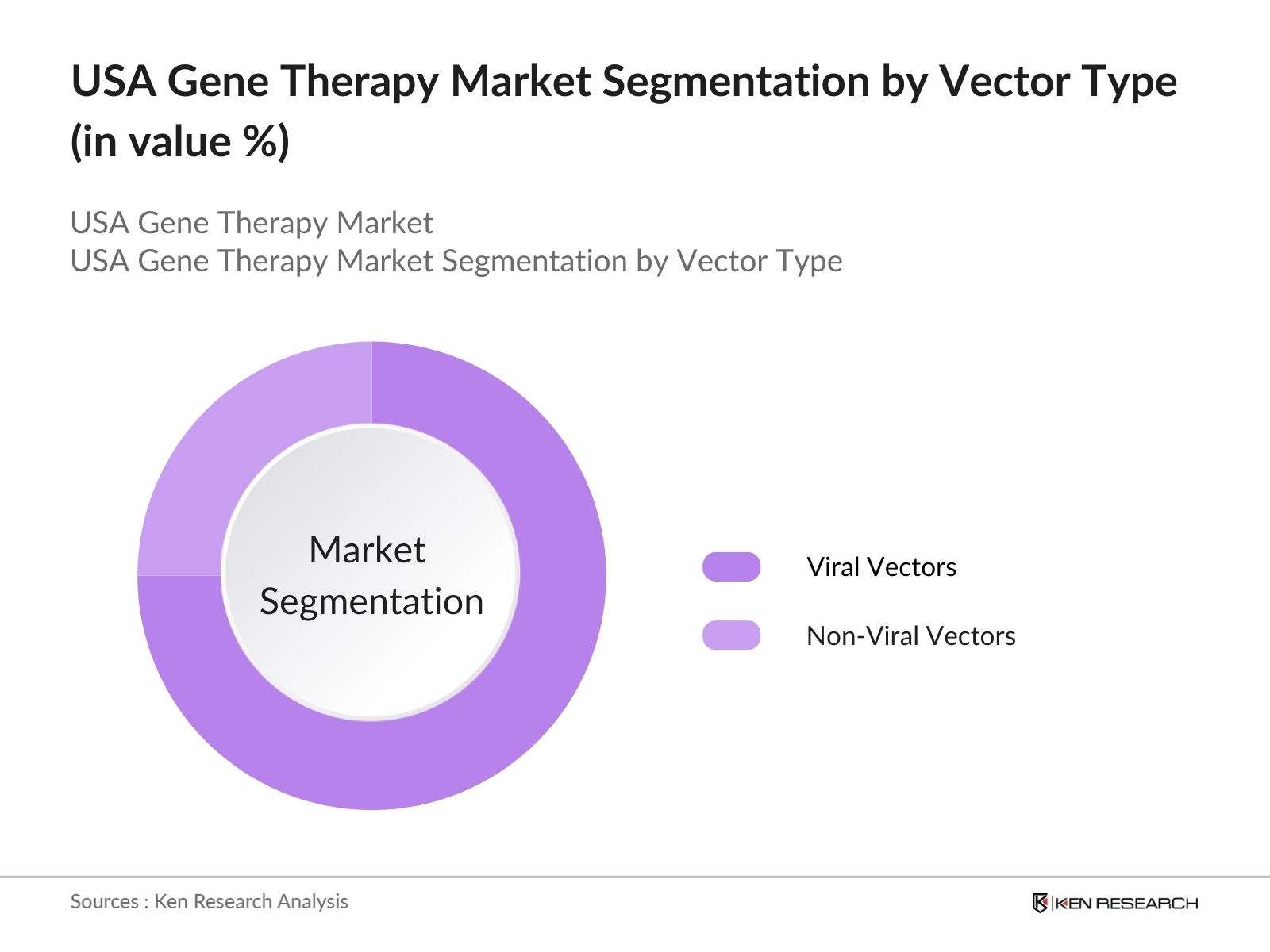
By Vector Type: The USA Gene Therapy market is segmented by vector type into viral vectors and non-viral vectors. Viral vectors, particularly adeno-associated viruses (AAV), have a dominant market share due to their efficacy in delivering genetic material to target cells. AAV vectors have been widely used in clinical trials for various genetic disorders, particularly in conditions such as hemophilia and spinal muscular atrophy. Their established safety profile and ability to target specific cells contribute to their prominence in the market.
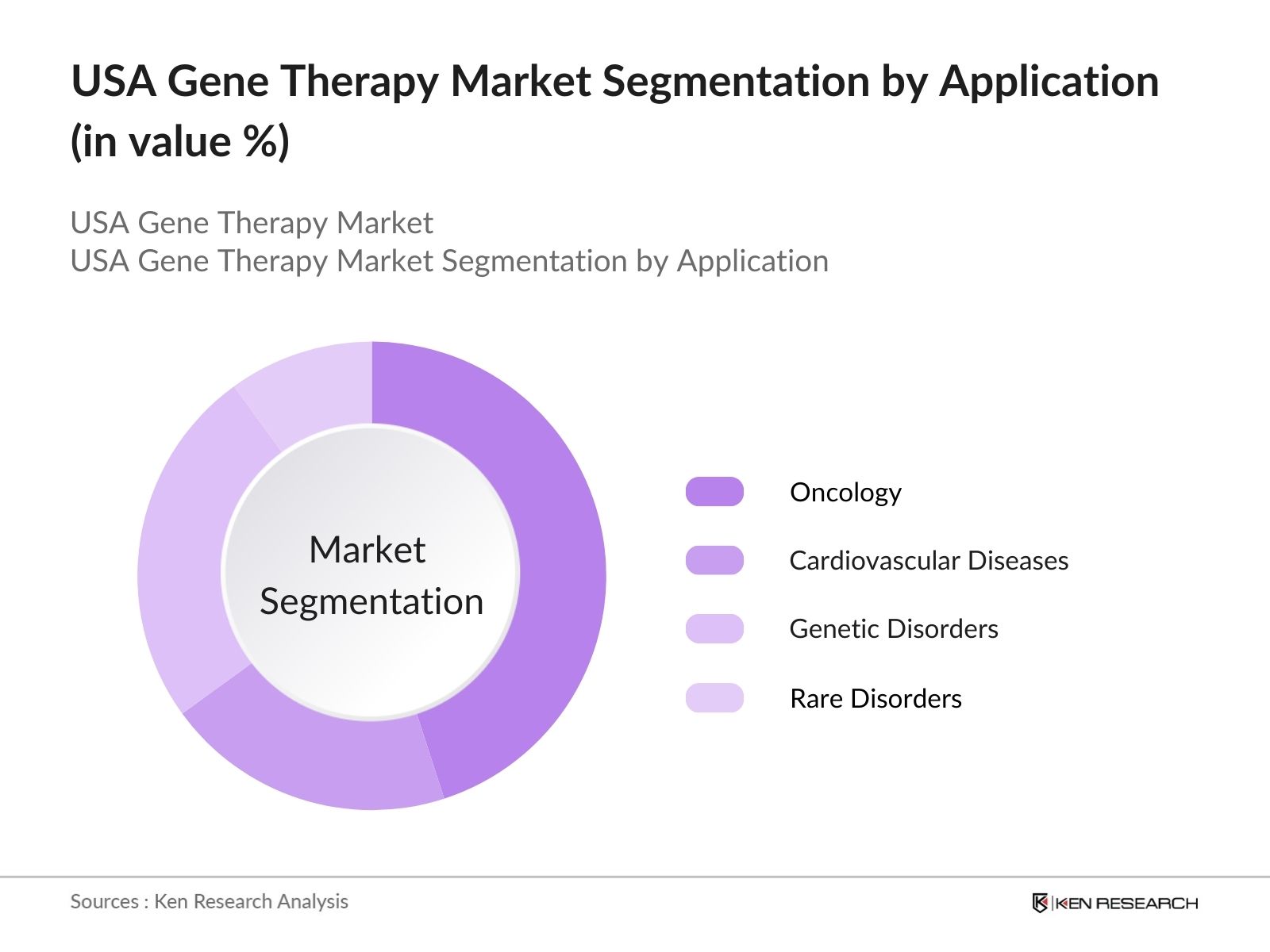
By Application: The USA Gene Therapy market is also segmented by application into oncology, cardiovascular diseases, genetic disorders, and rare diseases. Oncology holds the largest market share due to the growing incidence of cancers and the urgent need for targeted treatments. Gene therapy offers a promising solution for treating cancer by modifying genetic material to either enhance the bodys immune response or directly attack cancer cells. Treatments such as CAR-T therapy have shown significant success in clinical trials, leading to their growing adoption.
USA Gene Therapy Market Competitive Landscape
The USA Gene Therapy market is characterized by the presence of both established pharmaceutical giants and innovative biotech companies. The market is competitive, with companies constantly investing in research and development to bring new therapies to market. Leading players in the gene therapy space are distinguished by their extensive product pipelines, collaboration with academic institutions, and early entry into clinical trials. The market's competitive nature highlights the strategic importance of clinical trial success, patent portfolios, and collaborations with research institutes.
|
Company |
Establishment Year |
Headquarters |
Revenue |
Number of Employees |
R&D Spending |
Product Pipeline |
Patent Portfolio |
Collaborations |
|
Pfizer Inc. |
1849 |
New York, USA |
- |
- |
- |
- |
- |
- |
|
Novartis AG |
1996 |
Basel, Switzerland |
- |
- |
- |
- |
- |
- |
|
Bluebird Bio, Inc. |
2010 |
Cambridge, USA |
- |
- |
- |
- |
- |
- |
|
RegenxBio Inc. |
2008 |
Maryland, USA |
- |
- |
- |
- |
- |
- |
|
Sarepta Therapeutics |
1980 |
Cambridge, USA |
- |
- |
- |
- |
- |
- |
USA Gene Therapy Market Analysis
Growth Drivers
- Increased R&D in Genetic Disorders: The USA is experiencing a surge in R&D spending for genetic disorders, particularly in the area of gene therapy. In 2023, the National Institutes of Health (NIH) allocated over $41 billion towards biomedical research, with a notable portion directed to genetic disorder studies. For instance, the prevalence of cystic fibrosis, a common genetic disorder, affects about 30,000 people in the U.S., driving investment in gene therapies. The NIHs dedicated funding towards genetic research has seen year-on-year increases, fueling developments in gene therapy.
- Expanding Regulatory Approvals: As of early 2024, the FDA has approved a total of35 gene therapy products. In the previous year,seven new gene therapieswere approved, which aligns with the ongoing trend of increasing approvals in this area. Breakthrough Therapy designations, which expedite development, have increased significantly. In 2023 alone, the FDA granted 17 gene therapy-related Breakthrough Therapy designations. These approvals are pivotal for commercializing gene therapies for previously untreatable diseases like spinal muscular atrophy.
- Increasing Precision Medicine Adoption: The All of Us Research Program has released whole-genome sequence data from 245,388 participants, with 77% from communities historically underrepresented in biomedical research and 46% from racial and ethnic minorities. The rise in genomic data availability through projects like the All of Us Research Program, which gathered over one million genomic data sets, has enhanced gene therapys precision. This surge in personalized medicine is directly boosting gene therapy adoption.
Challenges
- High Treatment Costs: Gene therapy treatments, while revolutionary, face high costs. In 2023, the cost per patient for some gene therapies exceeded $2 million, posing challenges for widespread adoption. The expensive production process, driven by complex manufacturing and R&D costs, further contributes to these pricing hurdles. As gene therapies advance, the financial burden remains a significant challenge for healthcare providers and patients alike.
- Regulatory and Ethical Hurdles: Navigating FDA regulations and ethical concerns remains a challenge for gene therapy in the U.S. The FDAs rigorous approval process for gene therapies, while ensuring safety, can delay market entry. Moreover, ethical concerns surrounding germline gene editing, highlighted by debates in the bioethics community, have slowed some gene therapy applications. Ethical frameworks are still being developed, leading to uncertainty in regulatory pathways.
USA Gene Therapy Future Market Outlook
Over the next five years, the USA Gene Therapy market is expected to experience substantial growth, fueled by ongoing advancements in gene-editing technologies such as CRISPR, a rise in the number of clinical trials for gene therapies, and increasing regulatory support. The expanding application of gene therapy across various medical conditions, particularly in oncology and rare diseases, will also drive the market forward.
Key factors contributing to future growth include technological advancements in vector delivery systems, increased healthcare spending, and a growing understanding of genetic diseases, enabling more personalized treatment approaches.
Market Opportunities
- Gene Editing Advancements: There have indeed been over 250 clinical trials involving CRISPR-based gene editing technologies, particularly focusing on conditions such as sickle cell anemia. This reflects the growing interest and investment in CRISPR as a viable method for treating genetic disorders. The expanding toolkit of gene-editing technologies presents an opportunity to enhance precision and efficacy in gene therapies, opening new treatment avenues.
Source: NIH Clinical Trials Database - Expansion of Orphan Drug Market: The Orphan Drug Act has indeed facilitated the development of therapies for rare diseases by providing incentives for pharmaceutical companies. In 2023, the FDA approvedElevidys(delandistrogene moxeparvovec), marking it as the first gene therapy for DMD. The FDAs incentives for orphan drug development, including tax credits and market exclusivity, offer a lucrative opportunity for companies in the gene therapy space.
Scope of the Report
|
Segment |
Sub-Segments |
|
Vector Type |
Viral Vectors |
|
Non-Viral Vectors |
|
|
Application |
Oncology |
|
Cardiovascular Diseases |
|
|
Genetic Disorders |
|
|
Rare Diseases |
|
|
Therapy Type |
Ex-Vivo Gene Therapy |
|
In-Vivo Gene Therapy |
|
|
Mode of Delivery |
Systemic Delivery |
|
Targeted Delivery |
|
|
Region |
Northeast USA |
|
Midwest USA |
|
|
Southern USA |
|
|
Western USA |
Products
Key Target Audience
Gene Therapy Product Manufacturers
Biopharmaceutical Companies
Hospitals and Healthcare Institutions
Biotechnology Companies
Gene Therapy-Focused Biotech Firms
Insurance Companies and Healthcare Payers
Investors and Venture Capitalist Firms
Government and Regulatory Bodies (FDA, NIH)
Companies
Major Players Mentioned in the Report
Pfizer Inc.
Novartis AG
Bluebird Bio, Inc.
RegenxBio Inc.
Sarepta Therapeutics
UniQure N.V.
Gilead Sciences, Inc.
Orchard Therapeutics
CRISPR Therapeutics
Editas Medicine
Intellia Therapeutics
Spark Therapeutics
Sangamo Therapeutics
Rocket Pharmaceuticals
Poseida Therapeutics
Table of Contents
1. USA Gene Therapy Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. USA Gene Therapy Market Size (in USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. USA Gene Therapy Market Analysis
3.1. Growth Drivers
3.1.1. Increased R&D in Genetic Disorders (R&D Spending, Genetic Disease Prevalence)
3.1.2. Expanding Regulatory Approvals (FDA Approvals, Breakthrough Therapies)
3.1.3. Increasing Precision Medicine Adoption (Personalized Medicine Metrics)
3.1.4. Rising Investments in Biotechnology (Investment Metrics, Biotechnology Developments)
3.2. Market Challenges
3.2.1. High Treatment Costs (Cost Per Patient, Pricing Challenges)
3.2.2. Regulatory and Ethical Hurdles (FDA Regulations, Ethical Concerns)
3.2.3. Complex Manufacturing Processes (Supply Chain, Manufacturing Bottlenecks)
3.3. Opportunities
3.3.1. Gene Editing Advancements (CRISPR, Gene-Editing Technologies)
3.3.2. Expansion of Orphan Drug Market (Orphan Drug Approvals, Market Potential)
3.3.3. Growing Collaboration with Academia (Partnerships with Universities, Research Initiatives)
3.4. Trends
3.4.1. Emerging Viral Vector Technologies (AAV, Lentivirus Utilization)
3.4.2. Increasing Focus on Rare Diseases (Rare Disease Pipeline, Clinical Trials)
3.4.3. Development of Non-Viral Gene Therapy (Non-Viral Delivery Methods)
3.5. Government Regulation
3.5.1. FDA Gene Therapy Regulatory Pathways (Breakthrough Therapy, RMAT)
3.5.2. Gene Therapy-related Intellectual Property (Patent Landscape)
3.5.3. Reimbursement and Insurance Coverage (Healthcare Insurance Policies, Reimbursement Guidelines)
3.6. SWOT Analysis
3.7. Stakeholder Ecosystem
3.8. Porters Five Forces
3.9. Competition Ecosystem
4. USA Gene Therapy Market Segmentation
4.1. By Vector Type (In Value %)
4.1.1. Viral Vectors
4.1.2. Non-Viral Vectors
4.2. By Application (In Value %)
4.2.1. Oncology
4.2.2. Cardiovascular Diseases
4.2.3. Genetic Disorders
4.2.4. Rare Diseases
4.3. By Therapy Type (In Value %)
4.3.1. Ex-Vivo Gene Therapy
4.3.2. In-Vivo Gene Therapy
4.4. By Mode of Delivery (In Value %)
4.4.1. Systemic Delivery
4.4.2. Targeted Delivery
4.5. By Region (In Value %)
4.5.1. Northeast USA
4.5.2. Midwest USA
4.5.3. Southern USA
4.5.4. Western USA
5. USA Gene Therapy Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Pfizer Inc.
5.1.2. Novartis AG
5.1.3. Gilead Sciences, Inc.
5.1.4. Bluebird Bio, Inc.
5.1.5. Orchard Therapeutics
5.1.6. Sarepta Therapeutics
5.1.7. RegenxBio Inc.
5.1.8. Editas Medicine
5.1.9. CRISPR Therapeutics
5.1.10. Intellia Therapeutics
5.1.11. Sangamo Therapeutics
5.1.12. Spark Therapeutics
5.1.13. UniQure N.V.
5.1.14. Rocket Pharmaceuticals
5.1.15. Poseida Therapeutics
5.2. Cross Comparison Parameters (Revenue, Headquarters, R&D Investments, Number of Patents, Product Pipeline, Employee Count, Inception Year, Market Share)
5.3. Market Share Analysis
5.4. Strategic Initiatives
5.5. Mergers and Acquisitions
5.6. Investment Analysis
5.7. Venture Capital Funding
5.8. Government Grants
5.9. Private Equity Investments
6. USA Gene Therapy Market Regulatory Framework
6.1. FDA Approval Process
6.2. Compliance Requirements
6.3. Safety and Efficacy Standards
6.4. GMP Guidelines for Gene Therapy
7. USA Gene Therapy Future Market Size (in USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. USA Gene Therapy Future Market Segmentation
8.1. By Vector Type (In Value %)
8.2. By Application (In Value %)
8.3. By Therapy Type (In Value %)
8.4. By Mode of Delivery (In Value %)
8.5. By Region (In Value %)
9. USA Gene Therapy Market Analysts Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Disclaimer
Contact Us
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves constructing an ecosystem map encompassing all major stakeholders within the USA Gene Therapy Market. This step is underpinned by extensive desk research, utilizing secondary and proprietary databases to gather comprehensive industry-level information. The primary objective is to identify and define the critical variables that influence market dynamics.
Step 2: Market Analysis and Construction
In this phase, we compile and analyze historical data pertaining to the USA Gene Therapy Market. This includes assessing market penetration, the ratio of gene therapy products to patients, and the resultant revenue generation. Additionally, service quality statistics are evaluated to ensure the reliability and accuracy of revenue estimates.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses are developed and subsequently validated through computer-assisted telephone interviews (CATIs) with industry experts from various companies. These consultations provide valuable insights into the operational and financial aspects of the gene therapy market.
Step 4: Research Synthesis and Final Output
The final phase involves direct engagement with multiple gene therapy manufacturers to acquire detailed insights into product segments, sales performance, consumer preferences, and other relevant factors. This interaction serves to verify and complement statistics derived from the bottom-up approach, ensuring a comprehensive, accurate analysis of the USA Gene Therapy Market.
Frequently Asked Questions
01. How big is the USA Gene Therapy Market?
The USA Gene Therapy Market is valued at USD 4 billion, driven by significant advancements in gene-editing technologies, the rising prevalence of genetic disorders, and increased regulatory approvals from the FDA.
02. What are the challenges in the USA Gene Therapy Market?
Challenges include high treatment costs, complex manufacturing processes, and ethical concerns surrounding genetic modifications. Additionally, the regulatory landscape is stringent, requiring extensive clinical trials and safety checks.
03. Who are the major players in the USA Gene Therapy Market?
Major players include Pfizer, Novartis, Bluebird Bio, Sarepta Therapeutics, and RegenxBio. These companies lead the market due to their strong product pipelines, extensive R&D investments, and strategic collaborations.
04. What are the growth drivers of the USA Gene Therapy Market?
Growth is driven by increased R&D investments, advancements in viral vector technologies, and the rising prevalence of genetic and rare diseases. Regulatory support through FDAs expedited approval pathways also boosts market growth.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.















