USA Molecular Diagnostics Industry outlook to 2030
Region:North America
Author(s):Dev Chawla
Product Code:KRO007
June 2025
90
About the Report
USA Molecular Diagnostics Market Overview
- The USA Molecular Diagnostics Market was valued at USD 5 billion, based on a five-year historical analysis. This growth is primarily driven by advancements in technology, increasing prevalence of chronic diseases, and the rising demand for personalized medicine. The market is also supported by the growing awareness of early disease detection and the need for rapid diagnostic solutions.
- Key players in this market include major cities such as New York, San Francisco, and Boston, which dominate due to their robust healthcare infrastructure, presence of leading research institutions, and a high concentration of biotechnology firms. These cities foster innovation and collaboration, making them hubs for molecular diagnostics development and commercialization.
- In 2023, the FDA implemented new regulations aimed at streamlining the approval process for molecular diagnostic tests. This initiative is designed to enhance patient access to innovative diagnostic tools while ensuring safety and efficacy, thereby promoting growth in the molecular diagnostics sector.
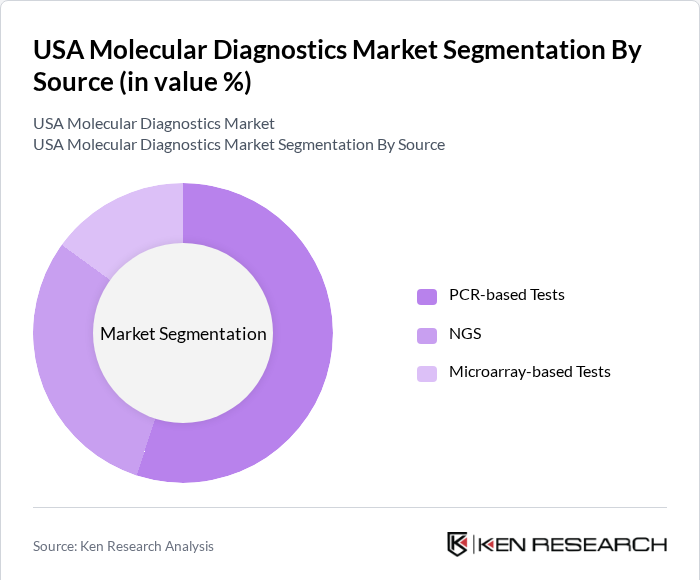
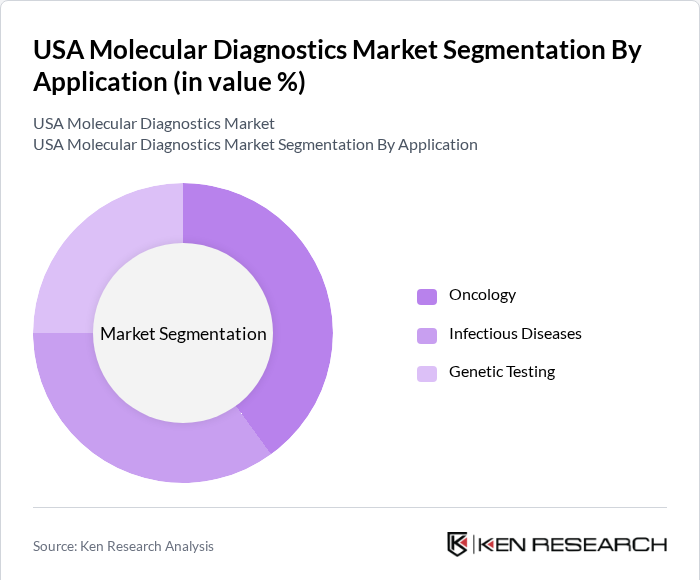
USA Molecular Diagnostics Market Segmentation
By Source: The molecular diagnostics market can be segmented into various sources, including PCR-based tests, NGS (Next-Generation Sequencing), and microarray-based tests. Among these, PCR-based tests dominate the market due to their widespread adoption in clinical laboratories for infectious disease detection and genetic testing. The reliability, speed, and cost-effectiveness of PCR technology have made it the preferred choice for healthcare providers, especially during the COVID-19 pandemic, which significantly increased the demand for rapid testing solutions.
By Application: The applications of molecular diagnostics include oncology, infectious diseases, genetic testing, and others. The oncology segment is currently leading the market, driven by the increasing incidence of cancer and the growing emphasis on personalized medicine. Molecular diagnostics play a crucial role in cancer detection, treatment selection, and monitoring, making them indispensable in oncology. The ability to provide targeted therapies based on genetic profiling is a significant factor contributing to the dominance of this segment.
USA Molecular Diagnostics Market Competitive Landscape
The USA Molecular Diagnostics Market is characterized by a competitive landscape featuring several key players, including Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Qiagen, and Illumina. These companies are engaged in continuous innovation and strategic partnerships to enhance their product offerings and maintain a competitive edge in the market.
 USA Molecular Diagnostics Market Industry Analysis
USA Molecular Diagnostics Market Industry Analysis
Growth Drivers
- Increasing Prevalence of Chronic Diseases: The USA has seen a significant rise in chronic diseases, with approximately 60% of adults living with at least one chronic condition as of 2024. This growing patient population drives demand for molecular diagnostics, which are essential for early detection and management. The Centers for Disease Control and Prevention (CDC) reported that chronic diseases account for 7 out of 10 deaths annually, highlighting the urgent need for advanced diagnostic solutions to improve patient outcomes.
- Advancements in Technology and Innovation: The molecular diagnostics sector is experiencing rapid technological advancements, particularly in areas like next-generation sequencing (NGS) and polymerase chain reaction (PCR) technologies. In 2024, the USA is projected to invest over $12 billion in R&D for diagnostic technologies, fostering innovation. These advancements enhance test accuracy and speed, making molecular diagnostics more accessible and effective, thus propelling market growth significantly.
- Rising Demand for Personalized Medicine: The shift towards personalized medicine is reshaping the healthcare landscape, with the molecular diagnostics market expected to benefit immensely. By 2024, the personalized medicine market is anticipated to reach $400 billion in the USA. Molecular diagnostics play a crucial role in tailoring treatments to individual genetic profiles, improving therapeutic efficacy and patient satisfaction, thereby driving market expansion in this segment.
Market Challenges
- High Costs Associated with Molecular Diagnostic Tests: The high costs of molecular diagnostic tests pose a significant barrier to widespread adoption. In 2024, the average cost of advanced molecular tests can exceed $1,800, limiting access for many patients and healthcare providers. This financial burden can deter healthcare systems from integrating these technologies, ultimately hindering market growth and patient access to essential diagnostic services.
- Regulatory Hurdles and Compliance Issues: The regulatory landscape for molecular diagnostics is complex, with stringent requirements from the FDA and other governing bodies. In 2024, the average time for regulatory approval can take up to 16 months, delaying product launches and innovation. These compliance challenges can discourage investment in new technologies, impacting the overall growth trajectory of the molecular diagnostics market in the USA.
USA Molecular Diagnostics Market Future Outlook
The future of the USA molecular diagnostics market appears promising, driven by ongoing technological advancements and an increasing focus on personalized healthcare solutions. As the healthcare system continues to evolve, the integration of artificial intelligence and machine learning into diagnostic processes is expected to enhance accuracy and efficiency. Furthermore, the expansion of point-of-care testing will likely improve accessibility, allowing for quicker diagnosis and treatment, ultimately benefiting patient care and market growth.
Market Opportunities
- Expansion of Point-of-Care Testing: The growing trend towards point-of-care testing presents a significant opportunity for the molecular diagnostics market. By 2024, the point-of-care testing segment is projected to grow, driven by the need for rapid and accurate diagnostics in various healthcare settings. This shift can enhance patient outcomes and streamline healthcare delivery, making it a vital area for investment and development.
- Growth in Genetic Testing and Precision Medicine: The increasing focus on genetic testing and precision medicine offers substantial growth potential. In 2024, the genetic testing market is expected to reach $25 billion in the USA, fueled by advancements in genomics and personalized therapies. This trend will likely drive demand for molecular diagnostics, as healthcare providers seek to tailor treatments based on individual genetic profiles, enhancing therapeutic effectiveness.
Scope of the Report
| By Source |
Polymerase Chain Reaction (PCR) Next-Generation Sequencing (NGS) Microarray-based tests |
| By Application |
Oncology Infectious Diseases Genetic Testing
|
| By Technology |
Polymerase Chain Reaction (PCR) Sequencing Technologies Microarray Technology Isothermal Nucleic Acid Amplification Technology |
| By End-User |
Hospitals Diagnostic Laboratories Research Institutions |
| By Region |
North America Europe Asia-Pacific Latin America Middle East & Africa |
Products
Key Target Audience
Investors and Venture Capitalist Firms
Government and Regulatory Bodies (e.g., Food and Drug Administration, Centers for Disease Control and Prevention)
Manufacturers and Producers
Distributors and Retailers
Healthcare Providers and Laboratories
Biotechnology and Pharmaceutical Companies
Industry Associations (e.g., American Clinical Laboratory Association)
Financial Institutions
Companies
Players Mentioned in the Report:
Roche Diagnostics
Abbott Laboratories
Thermo Fisher Scientific
Qiagen
Illumina
Genomic Health Innovations
Precision Diagnostics Solutions
BioMolecular Insights
NextGen Pathways
Clarity Molecular Technologies
Table of Contents
1. USA Molecular Diagnostics Market Overview
1.1. Definition and Scope
1.2. Market Taxonomy
1.3. Market Growth Rate
1.4. Market Segmentation Overview
2. USA Molecular Diagnostics Market Size (In USD Bn)
2.1. Historical Market Size
2.2. Year-On-Year Growth Analysis
2.3. Key Market Developments and Milestones
3. USA Molecular Diagnostics Market Analysis
3.1. Growth Drivers
3.1.1. Increasing prevalence of chronic diseases
3.1.2. Advancements in technology and innovation
3.1.3. Rising demand for personalized medicine
3.2. Market Challenges
3.2.1. High costs associated with molecular diagnostic tests
3.2.2. Regulatory hurdles and compliance issues
3.2.3. Limited reimbursement policies
3.3. Opportunities
3.3.1. Expansion of point-of-care testing
3.3.2. Growth in genetic testing and precision medicine
3.3.3. Increasing investments in research and development
3.4. Trends
3.4.1. Integration of artificial intelligence in diagnostics
3.4.2. Shift towards decentralized testing solutions
3.4.3. Growing focus on infectious disease diagnostics
3.5. Government Regulation
3.5.1. Overview of FDA regulations for molecular diagnostics
3.5.2. Impact of CLIA on laboratory testing
3.5.3. Compliance with HIPAA for patient data protection
3.5.4. Role of CMS in reimbursement policies
3.6. SWOT Analysis
3.7. Stake Ecosystem
3.8. Porter’s Five Forces
3.9. Competition Ecosystem
4. USA Molecular Diagnostics Market Segmentation
5. USA Molecular Diagnostics Market Competitive Analysis
5.1. Detailed Profiles of Major Companies
5.1.1. Roche Diagnostics
5.1.2. Abbott Laboratories
5.1.3. Thermo Fisher Scientific
5.1.4. Qiagen
5.1.5. Illumina
5.1.6. Genomic Health Innovations
5.1.7. Precision Diagnostics Solutions
5.1.8. BioMolecular Insights
5.1.9. NextGen Pathways
5.1.10. Clarity Molecular Technologies
5.2. Cross Comparison Parameters
5.2.1. Market Share Analysis
5.2.2. Product Portfolio Comparison
5.2.3. Geographic Presence
5.2.4. R&D Investment Levels
5.2.5. Customer Base Size
5.2.6. Pricing Strategies
5.2.7. Distribution Channels
5.2.8. Partnership and Collaboration Initiatives
6. USA Molecular Diagnostics Market Regulatory Framework
6.1. Environmental Standards
6.2. Compliance Requirements
6.3. Certification Processes
7. USA Molecular Diagnostics Market Future Market Size (In USD Bn)
7.1. Future Market Size Projections
7.2. Key Factors Driving Future Market Growth
8. USA Molecular Diagnostics Market Future Market Segmentation
8.1. By Source
8.1.1. Polymerase Chain Reaction (PCR)
8.1.2. Next-Generation Sequencing (NGS)
8.1.3. Microarray-based tests
8.2. By Application
8.2.1. Oncology
8.2.2. Infectious Diseases
8.2.3. Genetic Testing
8.2.4. Others
8.3. By Technology
8.3.1. Polymerase Chain Reaction (PCR)
8.3.2. Sequencing Technologies
8.3.3. Microarray Technology
8.3.4. Isothermal Nucleic Acid Amplification Technology
8.4. By End-User
8.4.1. Hospitals
8.4.2. Diagnostic Laboratories
8.4.3. Research Institutions
8.5. By Region
8.5.1. North America
8.5.2. Europe
8.5.3. Asia-Pacific
8.5.4. Latin America
8.5.5. Middle East & Africa
9. USA Molecular Diagnostics Market Analysts’ Recommendations
9.1. TAM/SAM/SOM Analysis
9.2. Customer Cohort Analysis
9.3. Marketing Initiatives
9.4. White Space Opportunity Analysis
Research Methodology
Step 1: Identification of Key Variables
The initial phase involves constructing an ecosystem map encompassing all major stakeholders within the USA Molecular Diagnostics Market. This step is underpinned by extensive desk research, utilizing a combination of secondary and proprietary databases to gather comprehensive industry-level information. The primary objective is to identify and define the critical variables that influence market dynamics.
Step 2: Market Analysis and Construction
In this phase, we will compile and analyze historical data pertaining to the USA Molecular Diagnostics Market. This includes assessing market penetration, the ratio of marketplaces to service providers, and the resultant revenue generation. Furthermore, an evaluation of service quality statistics will be conducted to ensure the reliability and accuracy of the revenue estimates.
Step 3: Hypothesis Validation and Expert Consultation
Market hypotheses will be developed and subsequently validated through computer-assisted telephone interviews (CATIs) with industry experts representing a diverse array of companies. These consultations will provide valuable operational and financial insights directly from industry practitioners, which will be instrumental in refining and corroborating the market data.
Step 4: Research Synthesis and Final Output
The final phase involves direct engagement with multiple manufacturers to acquire detailed insights into product segments, sales performance, consumer preferences, and other pertinent factors. This interaction will serve to verify and complement the statistics derived from the bottom-up approach, thereby ensuring a comprehensive, accurate, and validated analysis of the USA Molecular Diagnostics Market.
Frequently Asked Questions
01. How big is the USA Molecular Diagnostics Market?
The USA Molecular Diagnostics Market is valued at USD 5 billion, driven by factors such as increasing demand, technological advancements, and supportive government initiatives.
02. What are the key challenges in the USA Molecular Diagnostics Market?
Key challenges in the USA Molecular Diagnostics Market include intense competition, regulatory complexities, and infrastructure limitations affecting market dynamics.
03. Who are the major players in the USA Molecular Diagnostics Market?
Major players in the USA Molecular Diagnostics Market include Roche Diagnostics, Abbott Laboratories, Thermo Fisher Scientific, Qiagen, Illumina, among others.
04. What are the growth drivers for the USA Molecular Diagnostics Market?
The primary growth drivers for the USA Molecular Diagnostics Market are increasing consumer demand, favorable policies, innovation, and substantial investment inflows.
Why Buy From Us?

What makes us stand out is that our consultants follows Robust, Refine and Result (RRR) methodology. i.e. Robust for clear definitions, approaches and sanity checking, Refine for differentiating respondents facts and opinions and Result for presenting data with story

We have set a benchmark in the industry by offering our clients with syndicated and customized market research reports featuring coverage of entire market as well as meticulous research and analyst insights.

While we don't replace traditional research, we flip the method upside down. Our dual approach of Top Bottom & Bottom Top ensures quality deliverable by not just verifying company fundamentals but also looking at the sector and macroeconomic factors.

With one step in the future, our research team constantly tries to show you the bigger picture. We help with some of the tough questions you may encounter along the way: How is the industry positioned? Best marketing channel? KPI's of competitors? By aligning every element, we help maximize success.

Our report gives you instant access to the answers and sources that other companies might choose to hide. We elaborate each steps of research methodology we have used and showcase you the sample size to earn your trust.

If you need any support, we are here! We pride ourselves on universe strength, data quality, and quick, friendly, and professional service.